The concept of acid-base titration
Acid-base titration is a titration analysis method based on acid-base reaction. It is one of the main and basic titration analysis methods. The essence of acid-base reaction is the reaction of proton transfer. According to the acid-base proton theory, all substances that can give protons are acids, and all substances that can accept protons are bases. For example, for the reaction of sodium hydroxide and hydrochloric acid, the reaction equation is: NaOH+HCL⇌NaCL+H2O. The substance that gives protons is hydrochloric acid (HCL), and the substance that receives protons is alkali: sodium hydroxide.Acid-base titration can be used to detect the content of acids, bases, and substances that can react with acids and bases. Therefore, this method is often used when measuring the pH of water. This method generally uses a strong acid or a strong base as a titrant. For example, HCL and H2SO4 are used as acid standard solutions to titrate alkaline substances, if CO2, H3PO4, etc. are used. To use the titration method to obtain accurate analysis results in water quality testing, it is necessary to master the selection of appropriate indicators to make the titration end point as close as possible to the measurement point.

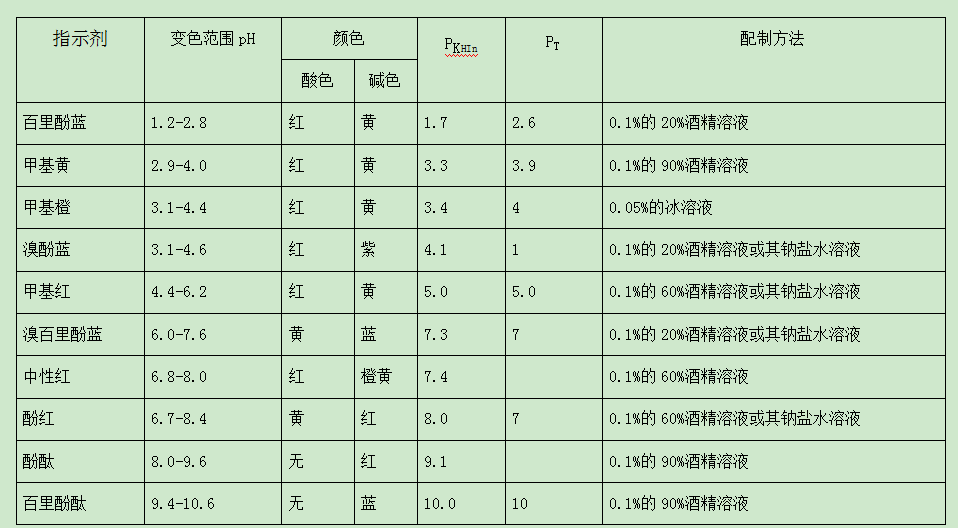
What are the commonly used acid-base indicators in water quality testing
In the process of acid-base titration, the solution itself does not change in appearance, so the sudden change in the color of the acid-base indicator is often used to indicate the end point. The acid-base indicator is a kind of organic weak acid (represented by the symbol HIn) or organic weak base (represented by the symbol InOH) with a complex structure, and it is also amphoteric. They have a common feature: when the concentration of H in the solution changes, the color can change. This is because the indicator itself can have several different structures under different pH conditions, and different structures show different colors. Therefore, when the pH of the solution changes, it causes a color change. For example, phenolphthalein is a very weak organic acid. If HIn is used to represent its molecule, the following ionization equilibrium exists in the solution: InOH⇌H+In.Phenolphthalein undergoes structural changes at the same time of ionization, that is, the structure of molecules and ions are different, so ionized ions and unionized molecules have different colors. The molecule HIn is colorless, while the ion In is red. If acid or alkali is added to the solution, it will affect the ionization balance between molecules and ions. In an acidic solution, due to the presence of a large amount of H, the balance shifts to the left, and phenolphthalein mainly exists in molecular form and is colorless; in alkali In the sexual solution, due to the decrease of H concentration, the above balance shifts to the right, and phenolphthalein mainly exists in the form of ions and is red.
Another example is methyl orange, which is an organic weak base. InOH represents its molecule, its molecule is yellow, and its ion is red. The following ionization equilibrium exists in the aqueous solution: InOH⇌OH+In
In an acidic solution, the equilibrium shifts to the right, and the solution appears red. In an alkaline solution, the equilibrium shifts to the left, and the solution appears yellow.
The discoloration principle of other acid-base indicators is similar to that of phenolphthalein and methyl orange. In fact, it is not that the pH value of the solution changes slightly to observe the color change of the indicator. It must be the pH value of the solution that changes to a certain range before the color change of the indicator can be observed. This range is the discoloration of the indicator. range.