Sulfate in water is a common substance, which is widely present in various natural water bodies. It is mainly formed by slowly permeating formation minerals into the groundwater. Therefore, the concentration of sulfate in the water quality of different environments is not the same. Before, we introduced the gravimetric method to detect sulfate in water, but this type of method is suitable for boiler water and industrial cold circulating water with a content of less than 10mg/L. To detect sulfate in natural water samples such as river water and lake water, we can use the barium chromate photometric method.
The principle is that in an acidic solution, barium chromate and sulfate form barium sulfate precipitation and release chromate ions. After the solution is neutralized, the excess barium chromate and the generated barium sulfate are still in a precipitated state, and the precipitate is removed by filtration. Under alkaline conditions, the chromate ion appears yellow, and the content of sulfate can be known by measuring its absorbance.

Instruments and reagents used in barium chromate photometry
1. Colorimetric tube 50mL
2. Erlenmeyer flask 250mL
3. Heating and filtering device
4. Spectrophotometer
5. Barium chromate suspension
Weigh 19.44g potassium chromate and 24.44g barium chloride, respectively dissolve them in 1L tertiary reagent water, and heat to boiling. Pour the two solutions into the same 3L beaker, at this time a yellow barium chromate precipitate is formed. After the precipitation has dropped, pour out the supernatant, and then wash the precipitate with about 1L of tertiary reagent water each time. It takes about 5 washes in total. Finally, add tertiary reagent water to 1L to make a suspension, and mix well before each use. Each 5mL barium chromate suspension can precipitate about 48mg sulfate.
6. Ammonia (1+1)
7. Hydrochloric acid solution (2.5mol/L)
8. Sulfate Standard Solution
Weigh 1.4786g premium grade pure anhydrous sodium sulfate or 1.8141g anhydrous potassium sulfate, dissolve it in a small amount of tertiary reagent water, put it in a 1000mL volumetric flask, and dilute to the mark.
Sulfate detection steps in water
1. Draw a working curve
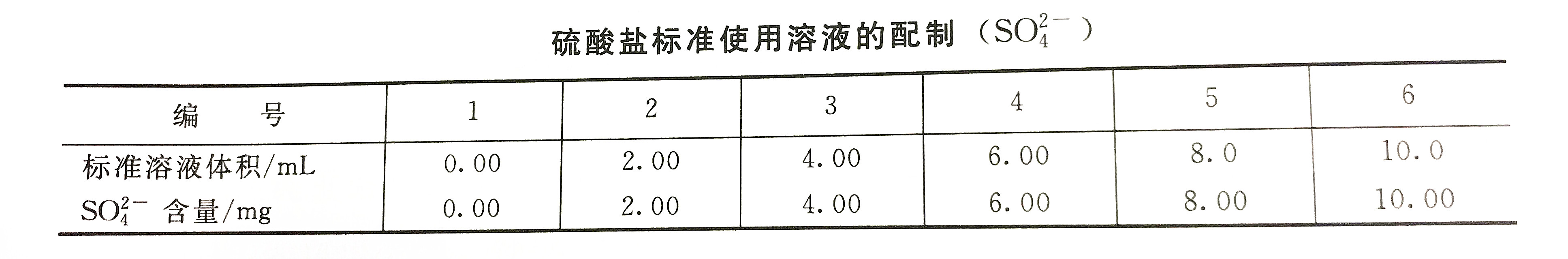
Take a set of solutions according to the standard solution preparation table for sulfates, pour them into a 150mL Erlenmeyer flask, and add three-grade reagent water to 50mL. Add 1 mL of 2.5moL hydrochloric acid solution to the bottle, heat and boil for about 5 minutes. After removing, add 2.5 mL of barium chromate suspension and boil for about 5 minutes. Remove the Erlenmeyer flask, after it is slightly cold, add (1+1) ammonia water dropwise to each flask until it is lemon-yellow, and then add 2 more drops. After the solution is cooled, filter it with a slow qualitative filter paper, and collect the filtrate in a 50mL colorimetric tube (if the filtrate is turbid, it should be repeatedly filtered until transparent). Wash the conical flask and filter paper three times with tertiary reagent water, collect the filtrate in the colorimetric tube, and dilute to the mark with tertiary reagent water. Then at the wavelength of 420nm, use a 10mm cuvette to measure the absorbance and draw a calibration curve.
2. Detect water samples
Take 50mL water sample, put it in a 150mL Erlenmeyer flask, add 1mL of 2.5mol/L hydrochloric acid solution, heat and boil for about 5min. Add 1 mL of 2.5mol/L hydrochloric acid solution to the water sample, heat and boil for about 5 minutes. After removal, add 2.5 mL of barium chromate suspension, and boil for about 5 minutes. Remove the Erlenmeyer flask, after it is slightly cold, add (1+1) ammonia water dropwise to the flask until it is lemon-yellow, and then add 2 more drops. After the solution is cooled, filter it with a slow qualitative filter paper, and collect the filtrate in a 50mL colorimetric tube (if the filtrate is turbid, it should be repeatedly filtered until transparent). Wash the conical flask and filter paper three times with tertiary reagent water, collect the filtrate in the colorimetric tube, and dilute to the mark with tertiary reagent water. Then at the wavelength of 420nm, the absorbance was measured with a 10mm cuvette, and finally the sulfate content in the water sample was obtained.
However, when testing, everyone should pay attention to the carbonate in the water sample can also form a precipitate with barium chromate, so before adding barium chromate to the water sample, it needs to be acidified and heated to remove carbonate.



