Most of the aluminum in daily drinking water comes from the alum used in the treatment. Under normal circumstances, the content of aluminum in natural water is actually very low. In addition, the "Sanitary Standards for Drinking Water" clearly stipulates that the content of heavy metal aluminum in drinking water shall not exceed 0.2mg/L. In fact, the detection of aluminum content in water is also very simple. Under acidic conditions, aluminum in water will form a complex with the reagent, and the content of aluminum in water can be obtained by detecting the absorbance of the complex. However, there are still many problems that need attention when testing the aluminum content in water.

Problems that need to be paid attention to when testing water samples
1. If the aluminum content of the water sample is large, take less water sample appropriately. Dilute to 50 mL with first-grade reagent water and then measure it according to the above method. At this time, the aluminum content of the water sample is the aluminum content found from the working curve multiplied by the dilution factor.
2. The iron in the water sample interferes with the determination. 1mg/L of iron will increase the measured value of aluminum by about 0.01mg/L. So when the iron content is greater than 100ug/L, the absorbance value of iron at 370nm should be deducted accordingly. After high iron is reduced to ferrous iron with hydroxylamine hydrochloride, it reacts with o-phenanthroline to form a stable complex. From the absorbance of the water sample at the wavelength of 370nm, the absorbance of the iron in the water sample at the wavelength of 370nm is subtracted to obtain the absorbance of the aluminum in the water sample at this wavelength. This absorbance can be used to quantify the aluminum content of the sample.
3. The complexation of fluoride ion with aluminum makes the result low. Other methods can be used when the interference is severe.
4. The fluoride ion in the water sample interferes with the determination. Beryllium sulfate can basically eliminate the interference of fluoride ions. But beryllium sulfate is toxic, so you must pay attention to safety when operating it.
5. Samples and standard samples cannot be treated with H2SO4.
6.When collecting water samples, special ground glass bottles should be used, and they should be soaked in hydrochloric acid (1+1) for more than 12 hours, and then thoroughly washed with first-class reagent water, and then added premium pure concentrated hydrochloric acid to the sampling bottle ( Add 2mL of concentrated hydrochloric acid to each 500mL water sample), take the water sample directly, and shake the water sample immediately.
7. When the water sample is turbid, take two water samples at the same time, and add 1.0mL citric acid solution, and blank one of them.
8. The content of orthophosphate and free chlorine in the water sample below 5mg/L will not interfere with the measurement.
9. The influence of other elements in the water sample on the measurement results is shown in the table below.
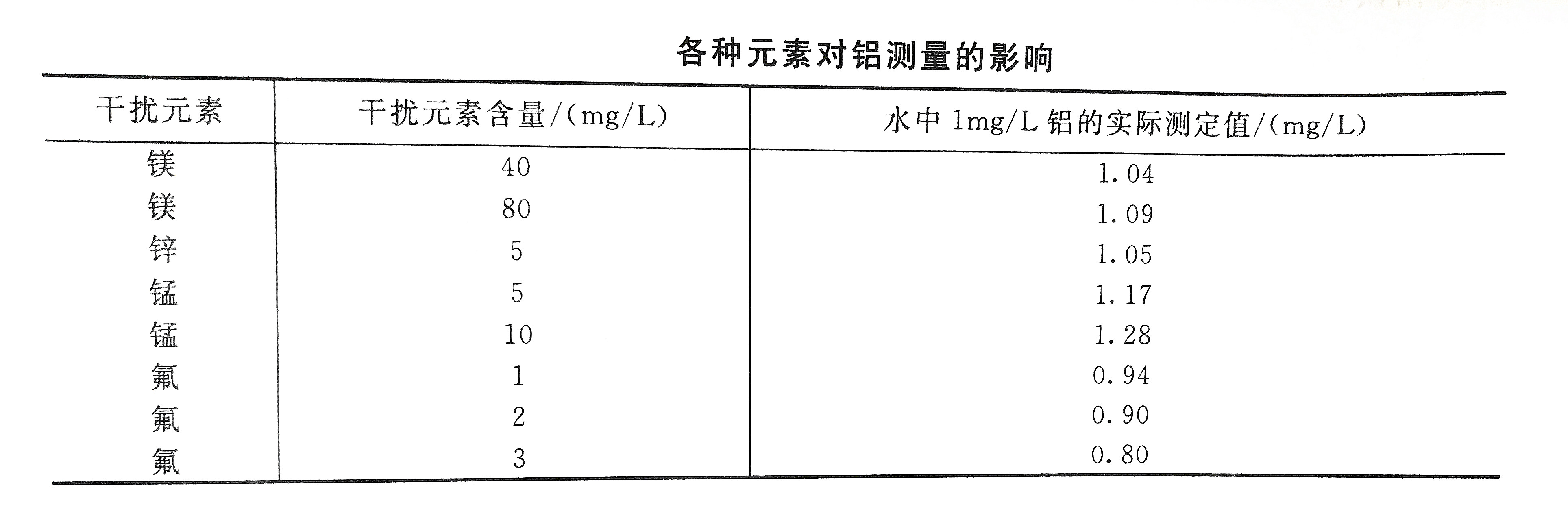
Problems that need to be paid attention to in the detection of aluminum content in water
1. This method is only applicable to water samples with low turbidity. If you want to determine a water sample with a large turbidity, the water sample should be acidified and filtered with a dense filter paper for determination.
2. Taking into account the color of the aluminum reagent itself, the amount of aluminum reagent added can be reduced to 1 mL when determining trace amounts of aluminum.
3. The temperature of the water sample should be similar to the temperature of the standard solution used when drawing the working curve (the difference is not more than ±5°C).
Problems that need to be paid attention to when testing reagents and drugs
1. Ascorbic acid is a reagent that can deteriorate easily when exposed to light, so please avoid light when using and storing.
2. The ascorbic acid and aluminum reagent solution can be stored in a brown bottle for one week.
The above are the issues that need to be paid attention to when testing the aluminum content in water



