As a common greenhouse gas, carbon dioxide is certainly not unfamiliar to everyone. Although it is colorless and odorless, it is also soluble in water. However, a large amount of carbon dioxide will form carbonic acid after being dissolved in water, which will affect the pH value of the water. A common application in life is carbonated beverages. Of course, there are few industries that can detect carbon dioxide in real life, but some companies do require the content of carbon dioxide in the water.
In this case, you can generally use the sodium hydroxide titration method to detect the carbon dioxide content in the water. This method is suitable for the measurement of freshwater and seawater samples with carbon dioxide content.

Collect and test water samples
1. Try to use glass bottles or polyethylene bottles when collecting water samples. Fill the bottles with the samples and tighten the caps. Do not agitate too much during sampling and mixing to avoid the volatilization of carbon dioxide.
2. Check the scale of the conical flask, burette and other sampling utensils. You can pour 100ml of pure water into the measuring cylinder after measuring, and then pour it into the conical flask to see if the scales are consistent. If something is different, replace the Erlenmeyer flask as soon as possible.
3. In order to ensure the accuracy of the detection data, the measurement should be performed as soon as possible after the collection is completed. The sample can be stored for 24 hours in an environment of 4°C. After this time, it needs to be re-sampled. In addition, the water sample needs to be returned to room temperature when testing after low-temperature storage.
4. When sampling, make the water slowly flow into the sampling bottle, and do not let air bubbles enter the water sampling bottle.
5. The sampling barrels and bottles used during collection should be cleaned with laboratory pure water before use.
Instruments and reagents used in testing
1.Erlenmeyer flask
2.Sampling barrel or bottle
3.Burette or digital titrator
4.Phenolphthalein indicator
5.Sodium hydroxide titrant
6.pH meter
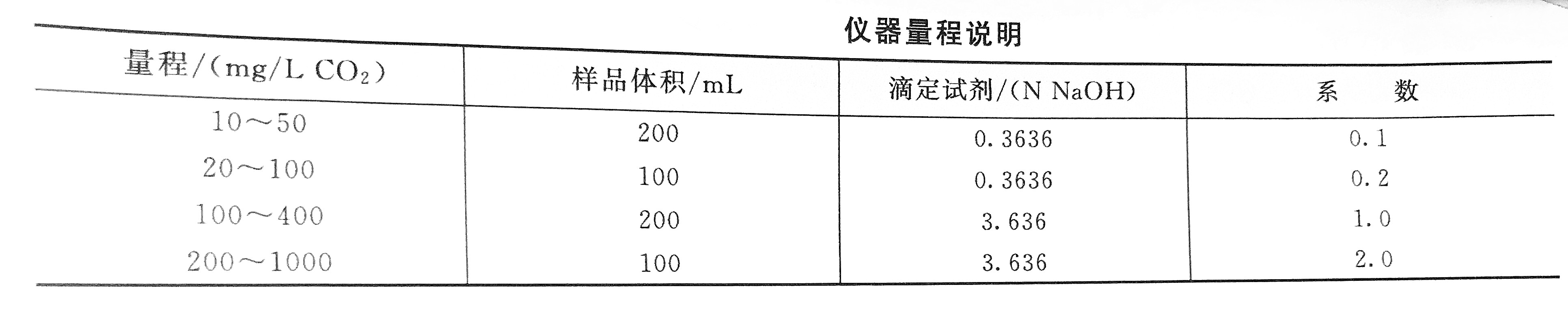
Detection steps
1. Prepare a suitable titrant in accordance with relevant standards.
2. Pour the collected water sample slowly into the Erlenmeyer flask to the standard mark.
3. Add the prepared phenolphthalein indicator to the water sample and mix well. If the water sample is pink, it means that there is no carbon dioxide in the water sample.
4. Put the burette into the water sample solution, drop the sodium hydroxide titrant while shaking, until the color of the water sample changes from colorless to light pink, and the color can be stored for 30 seconds without fading. Or use a pH meter to check the pH value of the solution. The water sample solution is the end point of the titration at pH 8.3. Record the amount of sodium hydroxide used, and use the correlation coefficient to calculate the concentration of carbon dioxide in the water sample.



