Pentachlorophenol is believed to be unfamiliar to many people. It is actually an organic compound. It was previously used as a herbicide for rice, and is currently mainly used for the preservation of wood, textiles and other items. Generally, the headspace solid-phase microextraction gas chromatography method is usually used to detect PCP in water. In equilibrium, the pentachlorophenol in the water will escape to the upper space and reach a dynamic equilibrium in the gas-liquid two-phase. At this time, the concentration of pentachlorophenol in the gas phase is proportional to its concentration in the liquid phase. The PCP in the gas phase was extracted with a solid-phase polypropionate extraction head for a certain period of time, and the samples were analyzed and injected in a gas chromatograph injector, and determined by an electron capture detector. According to the concentration of pentachlorophenol in the gas phase, the concentration of pentachlorophenol in the water sample can be calculated.

Reagents and instruments used for testing
1. Pure water
For pure water without pentachlorophenol, boil distilled water for 15min~30min or pass high-purity nitrogen gas for 20min~25min. Check that there are no chromatographic interference peaks before use.
2. Hydrochloric acid solution 1mol/L
Take 83mL of hydrochloric acid (p=1.18g/mL) and add laboratory pure water to dilute to 1L.
3. Sodium hydroxide solution 1mol/L
Weigh 0.04g of sodium hydroxide and dissolve it in 1L of laboratory pure water.
4. Sodium hydroxide solution 0.1mol/L
Weigh 4g of sodium hydroxide and dissolve it in 1L of laboratory pure water.
5. Pentachlorophenol standard material, chromatographically pure or certified reference material.
6. Sodium chloride.
7. Pentachlorophenol Standard Stock Solution
Accurately weigh 0.1000g of PCP standard substance, dissolve it with 0.1mol/L sodium hydroxide solution and dilute to 100mL. The concentration of this pentachlorophenol solution is 1000mg/L, and it can be stored at 4℃ for 1 month.
8. Pentachlorophenol standard solution
Take 1.00mL of the standard stock solution of pentachlorophenol, add it to a 100mL volumetric flask, dilute to volume with 1mmol/L sodium hydroxide solution, and then take 1.00mL of this solution and dilute to 100mL with 1mml/L sodium hydroxide solution. The concentration of pentachlorophenol in this standard solution is 0.100 mg/L.
9. High purity nitrogen > 99.999%
10. Gas chromatograph
11. Solid phase extraction device
12.100mL stoppered test tube or colorimetric tube
Water sample preservation and pretreatment
1. Bring a 100mL stoppered test tube or colorimetric tube to the collection site to take a 100mL water sample to be tested, add 1 mL of 0.1mol/L sodium hydroxide solution, and seal it. This water sample should be tested within 20 hours.
2. Take 5.00mL of the collected water sample and add it to the headspace bottle of 0.25mL of 1mol/L hydrochloric acid solution and 1.8g of sodium chloride in advance, and immediately seal the cap for testing.
Detection steps
1. Instrument parameter settings (reference)
a. Injector temperature: 280℃
b. Detector temperature: 300℃
c. Column flow rate 1.3mL/min
d. Split ratio: no split
e. The heating program of the column temperature is to increase from 100°C to 140°C at 10°C/min and hold for 2 minutes, and to increase to 200°C at 20°C/min and hold for 4 minutes.
2. Detection operation
Place the pretreated water sample on the solid-phase microextraction sampling stage and equilibrate at 60±1℃ for 40min. Insert the polyacrylate extraction head into the headspace bottle for adsorption for 12 minutes, take out the extraction head and insert it into the gas chromatograph injector at 280 °C for 2.5 minutes, and perform splitless injection for measurement.
draw standard curve
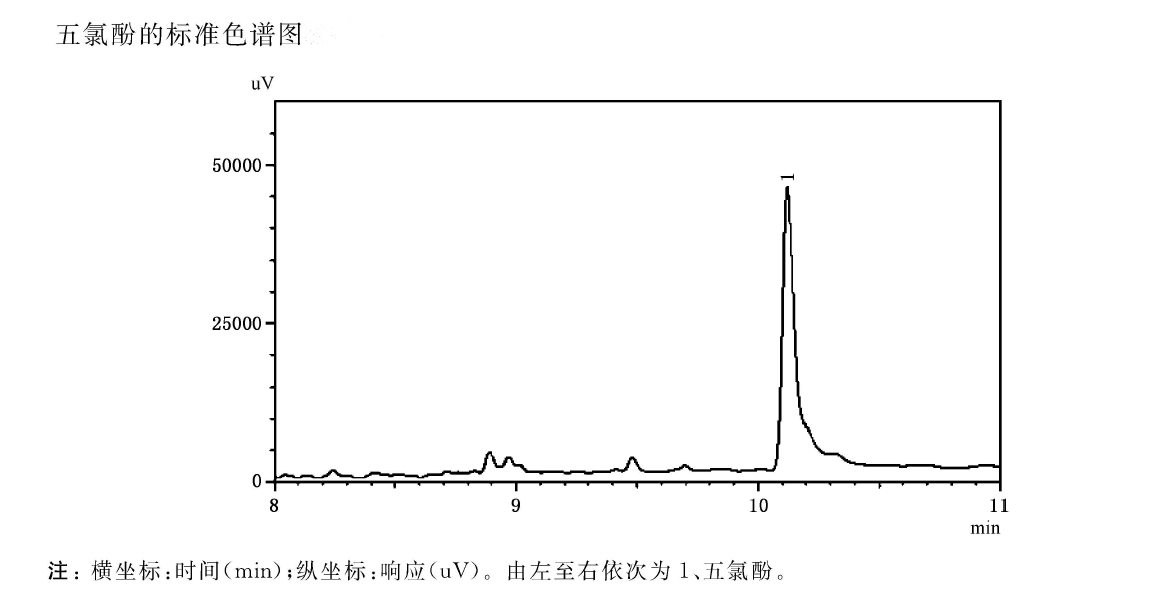
In the laboratory that does not contain pentachlorophenol or interfering substances in the air, take seven 100mL volumetric flasks and pipette 0mL, 0.20mL, 0.40mL, 0.80mL, 1.20mL, 1.60mL, 2.00mL standard solution of PCP respectively. , dilute with 1mmol/L sodium hydroxide solution to prepare standard series solutions of pentachlorophenol, the corresponding concentrations are 0.0ug/L, 0.20ug/L, 0.40ug/L, 0.80ug/L, 1.20ug/1, 1.60 ug/1, 2,00ug/L. Then draw 5.00mL of the prepared pentachlorophenol standard series solution into the headspace bottle pre-added with 0.25mL of 1mol/L hydrochloric acid solution and 1.8ug of sodium chloride, immediately seal the cap, and measure according to the detection steps. Draw a standard curve or calculate a regression equation with the concentration of the standard series solution versus the peak height value.
Finally, the properties of the components in the tested water sample were determined according to the peak retention time of the components in the standard chromatogram. The concentration of PCP was determined on the standard curve.



