The principle of ion chromatography to detect iodide in water is that the collected water sample enters the anion separation column along with the eluent, and the separated iodide ions are detected by a conductivity detector, qualitatively determined according to the retention time of iodide ions, and quantified by external standard method. When the water sample volume is 250ul, the detection limit of this method is 0.002mg/L.

Testing Instruments and Reagents
Instruments required for testing
1. Ion chromatograph (with conductivity detector).
2. Chromatographic column: anion separation column and anion guard column.
3. Continuous self-regenerating anion membrane suppressor or suppressor with equivalent performance.
4. Disposable syringe: 5ml.
5. Microporous membrane filter.
6. Polyethylene bottle or brown glass bottle: 500ml.
7. Cation exchange column, solid phase extraction column
8. Commonly used laboratory instruments and equipment
Reagents required for testing
1. Potassium iodide (KI): excellent grade pure, dried at 110℃ to constant weight before use.
2. Potassium hydroxide (KOH): excellent grade pure.
3. Sodium hydroxide (NaOH): excellent grade pure.
4. Iodide standard stock solution 1000mg/L
Weigh 1.3080g potassium iodide, dissolve in water and dilute to 1000ml; refrigerate at 4°C and store in the dark for one year. Or use commercially available certified standard solutions.
5. Iodide standard intermediate solution 100mg/L
Pipette 1000ml of iodide standard stock solution, dilute with water to 100m, and prepare for immediate use.
6. Iodide standard solution 10mg/L
Pipette 1000n iodide standard intermediate solution (55, dilute with water to 100m, and prepare for immediate use.
7. Potassium hydroxide solution 40 mmol/L
Weigh 22g8 potassium hydroxide (52), dissolve in water and dilute to 100n0 to mix, and store in a polyethylene bottle for later use.
8. Sodium hydroxide saturated solution
Dissolve an appropriate amount of solid sodium hydroxide in 100 ml of water at room temperature, stir to dissolve, and then continue to add solid sodium hydroxide and stir until a solid precipitates and no longer dissolves. After standing, take out the clear solution for later use.
9. High purity nitrogen.
10. Water system microporous membrane 0.45um
preservation of water samples
When collecting water samples for testing, polyethylene bottles or brown glass bottles are needed, and then a saturated sodium hydroxide solution is added to adjust the pH. The pH of the water sample was adjusted to 12. After the collection is completed, it should be tested as soon as possible. If it cannot be detected in time, it can be stored in the environment of 0-4 °C, and the storage time should not exceed 24 hours.
The collected water samples should be filtered through a 0.45um water-based microporous membrane, discard 10ml of the primary filtrate, and save the remaining filtrate for testing.

Specific detection steps
1. Reference conditions for chromatographic detection
Set up the instrument according to the instruction manual of the testing instrument. Chromatographic conditions are selected according to the chromatographic column. The reference conditions for isocratic elution using potassium hydroxide eluent system are as follows:
The eluent is 40mmol/L potassium hydroxide solution, the flow rate is 1.00ml/min, the suppressor current is 99mA, the detector temperature is 30℃, and the injection volume can be selected from 50ul-250ul according to the concentration of the sample.
2. Drawing of the standard curve
Draw water samples 0.00ml, 0.10ml, 0.20ml, 0.50ml, 1.00ml, 5.00ml, 10.00ml of iodide standard solution and place them in a set of 100ml volumetric flasks, dilute with water to the marked line and mix well, in the standard series The concentrations of iodide were: 0.000mg/L, 0.010mg/L, 0.020mg/L, 0.050mg/L, 0.100mg/L, 0.500mg/L, 1.00mg/L. Take the iodide concentration as the abscissa and its corresponding peak height or peak area as the ordinate to draw a standard curve.
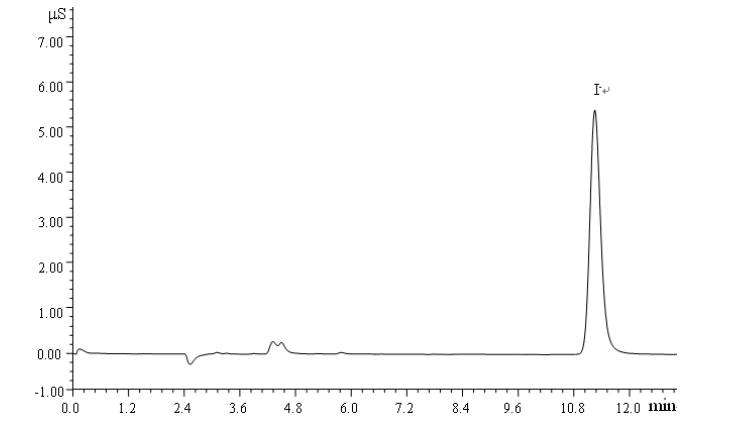
3. Detection steps
For the detection of water samples, the samples can be measured according to the same chromatographic conditions and steps for drawing the standard curve, and the retention time, peak area or peak height of the chromatographic peaks can be recorded. The concentration data of iodide in the water sample can be calculated according to the corresponding formula.
The above methods are derived from "HJ 778-2015 Water Quality Determination of Iodide Ion Chromatography"



