Glyphosate mentioned that this substance is believed to be familiar to many people who deal with the land. As a broad-spectrum organophosphorus herbicide, it can be seen in the north and south of my country. Although the emergence of glyphosate has greatly helped agricultural yields, its unreasonable abuse will damage water sources, soil, etc. Ecosystems cause pollution.
Glyphosate in water is generally detected by ion chromatography. The principle is that after the eluent is used to carry the water sample into the separation column, the glyphosate and the counterion with ion-exchange functional groups compete for the ion-exchange position on the column. Glyphosate was eluted from the column by continuous eluting of the eluent, and the separation was achieved. The conductivity value of the eluent is greatly reduced by the suppressor, and its chromatographic peak is obtained by the detector. The method is qualitative by retention time and quantitative by peak area. The detection limit of this method was 0.046 mg/L, and the lower limit of determination was 0.19 mg/L.
Reagents used for testing
1. Dechlorination agent: sodium thiosulfate, analytically pure.
2. Glyphosate reference material: certified reference material with a purity of more than 96%.
3. Sodium thiosulfate solution: 100 mg/L.
Take 0.1 g of sodium thiosulfate, dissolve it with laboratory first-grade pure water and dilute to 1 000 mL, and store it in a refrigerator below 4 ℃ in the dark.
4. Standard stock solution of glyphosate: 1000mg/L.
Accurately weigh 0.104 2 g glyphosate reference material, and weigh glyphosate reference materials of different purities according to the purity calibration, dissolve and dilute with laboratory first-grade pure water to a 100 mL volumetric flask, and prepare 1 000 mg/ L standard stock solution of glyphosate; sealed, refrigerated below 4 ℃ and protected from light, until use. This solution is valid for 30 days.
5. Glyphosate standard solution: 10 mg/L.
Accurately draw 1.00 mL glyphosate standard stock solution into a 100 mL volumetric flask, dilute to the mark with laboratory first-grade pure water, and prepare a 10 mg/L standard use solution. This solution is used now.
Instruments required for testing
1. Ion Chromatograph
With conductivity detector, eluent generator or equivalent, and anion suppressor.
2. Chromatographic column
Anion analysis column packing is ethylene ethyl styrene divinyl benzene copolymer; functional group is alkanol quaternary ammonium or alkyl quaternary ammonium; anion guard column packing is ethylene ethyl styrene divinyl benzene copolymer; or A Supp 5-150 Anion chromatography column (4 mm×150 mm) and 4/5 G guard column (4 mm×50 mm) or equivalent column.
3. Sample filter
Water-based nylon material, the pore size of the filter membrane is 0.45 μm.
4. Volumetric flask: 100 mL and 1 000 mL.
5. Analytical balance: 0.1 mg of sensitivity.
6. Glass single reticle pipette: 1 mL and 10 mL.
7. Graduated straw: 10 mL.
Detection steps
Testing instrument parameters
Hydroxide system instrument parameter settings:
Eluent flow rate: 1.0 mL/min;
Eluent concentration: 30 mmol/L, isocratic elution;
Suppressor current: 75 mA;
Injection volume: 100 μL.
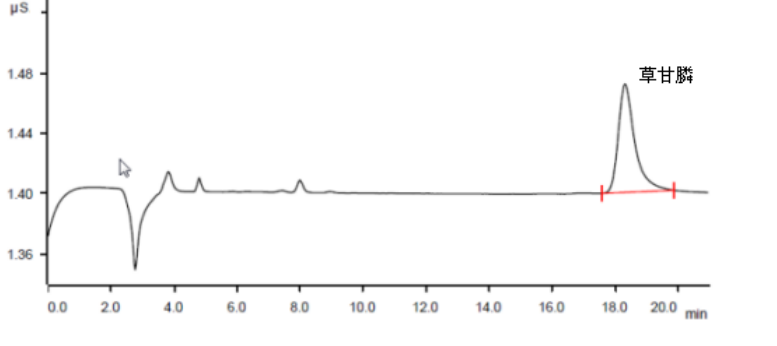
Carbonate system instrument parameter settings:
Eluent flow rate: 0.7 mL/min;
Eluent concentration: 8.0 mmol/L sodium carbonate solution;
Concentration of regeneration solution: 90 mmol/L sulfuric acid solution;
Injection volume: 100 μL.
Plotting the calibration curve
Pipette 0 mL, 2.00 mL, 4.00 mL, 6.00 mL, 8.00 mL and 10.00 mL standard glyphosate solution in a 100 mL volumetric flask with a graduated pipette respectively, and use laboratory first-grade pure water to prepare the concentration of glyphosate respectively. 0 mg/L, 0.20 mg/L, 0.40 mg/L, 0.60 mg/L, 0.80 mg/L and 1.00 mg/L standard series, this series of solutions are currently used and prepared. The standard series were measured from low to high, and the calibration curve was drawn with the peak area of glyphosate as the ordinate and the concentration as the abscissa.
Water sample determination
The treated sample was measured with an ion chromatograph, and the concentration of glyphosate was obtained from the calibration curve according to the peak area of glyphosate. If the concentration of glyphosate in the water sample exceeds the linear range of the calibration curve, the water sample should be diluted to within the linear range of the calibration curve and then measured.
Blank test
According to the detection operation steps, measure the pretreated blank sample and record the peak area.
Test result representation
Qualitative Analysis
Determine the glyphosate in the tested sample according to the retention time of glyphosate in the standard chromatogram.
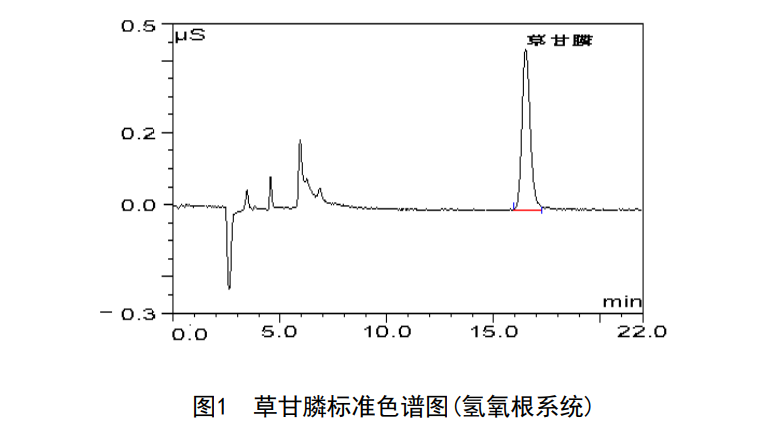
quantitative analysis
Quantitative analysis was performed according to the corresponding peak area of glyphosate on the chromatogram.
The concentration of glyphosate in water can be calculated according to the formula:
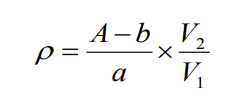
where:
ρ——the mass concentration of glyphosate in the water sample, in milligrams per liter (mg/L);
A - the peak area corresponding to glyphosate in the water sample (μS×min);
a — the slope of the calibration curve;
b — the intercept of the calibration curve;
V1 - volume of water sample (mL);
V2 - sample volume after dilution (mL).



