The organophosphorus pesticides in the water samples were extracted with chloroform, concentrated and fixed to volume, then separated by gas chromatography and detected by mass spectrometry. Trichlorfon in water samples was determined after being converted into dichlorvos by alkaline hydrolysis. Qualitative by retention time, mass spectrum or fragment ion mass-to-charge ratio and abundance ratio, and quantified by internal standard method.
Reagents for water sample testing
1. Trichloromethane: chromatographically pure, without interference peaks under the recommended conditions of the method.
2. Acetone: chromatographically pure, without interference peaks under the conditions recommended by the method.
3. Concentrated sulfuric acid: 1.84g/ml.
4. Sodium hydroxide.
5. Sodium chloride: After baking at 450℃ for 4 hours, put it in a desiccator to cool to room temperature, then put it in a reagent bottle and seal it for storage.
6. Anhydrous sodium sulfate: After baking at 450°C for 4 hours, put it in a desiccator to cool to room temperature, then put it in a reagent bottle and seal it for storage.
7. n-Hexane: chromatographically pure, no interference peaks under the recommended conditions of the method.
8. Sulfuric acid solution: 1+1.
9. Sulfuric acid solution: 1+10.
10. Sodium hydroxide solution: 10 g/L.
Weigh 1.0 g of sodium hydroxide, dissolve it in 100 ml of laboratory first-grade pure water, mix well, and store in a plastic reagent bottle with a screw mouth.
11. Sodium hydroxide solution: 1.0 g/L.
Weigh 1.0 g of sodium hydroxide, dissolve it in 1 L of laboratory first-grade pure water, mix well, and store it in a plastic reagent bottle with a screw mouth.
12. Organophosphorus pesticide standard stock solution (without trichlorfon): 2000g/ml.
Purchase commercially available certified standard solutions directly, or prepare them yourself. Commercially available certified reference materials are stored in accordance with the instructions; self-prepared standard stock solutions are stored frozen at ≤-18°C and can be stored stably for 6 months.
13. Standard stock solution of trichlorfon: 2000g/ml.
Purchase commercially available certified standard solutions directly, or prepare them yourself. Commercially available certified reference materials are stored in accordance with the instructions; self-prepared standard stock solutions are stored frozen at ≤-18°C and can be stored stably for 6 months.
14. Organophosphorus pesticide application solution: 50.0g/ml.
Take an appropriate amount of organophosphorus pesticide standard stock solution and prepare it in a certain volume of n-hexane:acetone (1:1).
15. Organophosphorus pesticide column treatment solution: 10.0g/ml.
Take an appropriate amount of the standard stock solution of organophosphorus pesticides and prepare it in a certain volume of n-hexane:acetone (1:1).
16. Internal standard standard stock solution: 2000g/ml.
Naphthalene-D8, acenaphthene-D10, phenanthrene-D10, and Qi-D12 should be selected as internal standards. Commercially available certified standard solutions can be purchased directly, or prepared by themselves. Commercially available certified reference materials are stored in accordance with the instructions; self-prepared internal standard standard stock solution is stored frozen at ≤-18°C, and can be stored stably for 12 months.
17. Substitute (deuterated tributyl phosphate) standard stock solution: 1000g/ml.
Commercially available certified standard solutions can be purchased directly, or prepared by themselves. Commercially available certified reference materials are stored in accordance with the requirements of the instructions; standard stock solutions of self-prepared substitutes are stored frozen at ≤-18°C, and can be stored stably for 6 months.
18. Substitute (deuterated tributyl phosphate) standard solution: 50.0g/ml.
Take an appropriate amount of the substitute (deuterated tributyl phosphate) standard stock solution and prepare it in a certain volume of n-hexane:acetone (1:1), and use it now.
19. Decafluorotriphenylphosphine (DFTPP) solution: 50 mg/L.
Commercially available certified standard solutions can be purchased directly, or prepared by themselves. Self-prepared standard solutions are stored frozen at ≤-18°C.
20. Helium: purity ≥99.999%.
Instruments used for testing
1. Gas chromatograph: with split/splitless injection port, electronic pressure control for carrier gas, programmable temperature rise.
2. Mass spectrometer: Electron bombardment ionization source (EI), which can scan from 35amu to 500amu in one second; with NIST mass spectrum library, manual/automatic tuning, data acquisition, quantitative analysis and spectral library retrieval functions.
3. Capillary column: Capillary column: 30m×0.25mm, film thickness, film thickness 0.25m, stationary phase is, stationary phase is 50% phenylphenyl/50% methyl polysiloxane, or use other methyl polysiloxane, or use other equivalent capillary columns. Capillary columns with efficient performance.
4. Concentrator concentrator::KD concentrator or other concentrator concentrator or other concentrator. .
5. Sample bottle: Brown ground stopper glass bottle or with PTFE Sample bottle: Brown ground stopper glass bottle or brown screw top glass bottle with PTFE liner cap liner cap. brown screw-on glass bottle.
6. Graphitized carbon black column Graphitized carbon black column: 250mg/3ml. .
7. Micro syringe: Micro syringe: 5ul, 10ul, 50ul, 100ul and 1ml. .
8. General laboratory instruments and equipment.
Precautions for water sample collection and preservation
After collecting the water sample, adjust the pH of the water sample to 5-8 in the water collector with sulfuric acid solution or sodium hydroxide solution, and transfer it to a brown ground stopper glass bottle or a brown screw top glass bottle with a teflon-lined bottle cap bottle.
For each batch of water samples, at least one blank sample of the whole procedure should be collected (replace the sample with the same batch of experimental water).
After collection, water samples should be refrigerated and transported away from light for timely analysis. If it cannot be analyzed in time, it should be stored in a refrigerator and protected from light, and the storage period is 3d.
Preparation of samples
Extraction and concentration of water samples
Surface water, groundwater and seawaterMeasure 1L of water sample into a separatory funnel and add 10.0ul of the standard stock solution of substituted tributyl phosphate to mix well. Add 30 g of sodium chloride to the water sample, shake until completely dissolved, add 25 ml of chloroform, shake for 2 minutes, and pay attention to degassing. After standing to separate the layers, the extract was transferred to the corresponding container. Repeat the extraction twice, combine the extracts, dehydrate the extracts with anhydrous sodium sulfate, collect them in a concentration bottle, concentrate them to near dryness, dilute to 1.0ml with n-hexane acetone 1:1, add 5.0ul of internal standard standard stock solution 4.16 to be tested . At the same time, keep the trichlorfon to be tested in the water layer.
Domestic and industrial wastewater
Measure 100ml of water sample into the separatory funnel, add 10.0ul of substitute tributyl phosphate standard solution 4.17 and mix well. Add 3 g of sodium chloride to the water sample, shake until completely dissolved, add 10 ml of chloroform, shake for 2 minutes, and pay attention to degassing. After standing to separate the layers, the extract was transferred to the corresponding container. Repeat the extraction twice, combine the extracts, dehydrate the extracts with anhydrous sodium sulfate, collect them in a concentration bottle, concentrate them to near dryness, dilute to 1.0ml with n-hexane acetone 1:1, and add 5.0ul of the internal standard standard stock solution to be tested. . At the same time, keep the trichlorfon to be tested in the water layer.
Purification and concentration of water samples
For domestic sewage and industrial wastewater with complex components, or when the color of the extract is dark, the extract can be purified. The graphitized carbon black cartridge was activated with 5ml n-hexane:acetone (1:1) in advance, and then 1.0ml of the extract without the internal standard solution was added to the activated graphitized carbon black cartridge, followed by 10ml n-hexane. Elution with hexane:acetone (1:1), collect all the eluents that have passed through the column, concentrate to dryness, dilute to 1.0ml with n-hexane:acetone (1:1), add 5.0ul internal standard standard stock solution, wait for Measurement.
Preparation of Trichlorfon Samples
Adjust the pH of the collected water layer from surface water, groundwater, seawater, domestic sewage and industrial wastewater to 9-10 with sodium hydroxide solution, pour it into a conical flask, close the cap, and place it in a water bath at 50°C Alkaline hydrolysis was carried out in the flask, and the conical flask was shaken continuously. After 15 minutes, take out the conical flask, cool it to room temperature, adjust the pH to 5-6 with sulfuric acid solution, transfer the solution to a separatory funnel, and then perform extraction and concentration according to the steps.
Water sample testing steps
Instrument Reference Conditions
Gas Chromatography Reference Conditions
Injection port: temperature 220℃, splitless;
Carrier gas: helium;
Column flow: 1.0ml/min (constant flow);
Column temperature: 40 °C for 4 min; to 270 °C (12 min).
MS reference conditions
Transmission line temperature: 270℃;
Ion source temperature: 230℃;
Ion source electron energy: 70eV;
Data collection method: full scan;
Scan quality range: 35amu-500amu.
Instrument performance check
Pipette 1ul-2ul decafluorotriphenylphosphine (DFTPP) solution with a micro-syringe and inject it directly into the gas chromatograph for analysis. The obtained decafluorotriphenylphosphine (DFTPP) key ion abundance should be consistent with decafluorotriphenylphosphine (DFTPP). (DFTPP) key ion abundance standard, otherwise it is necessary to adjust the parameters of the mass spectrometer or consider cleaning the ion source. If the instrument software cannot automatically determine whether the abundance of the key ions of decafluorotriphenylphosphine (DFTPP) meets the standard, the key ions can be obtained by taking the average of the ion abundances of the peak top scan point and the two scan points before and after deducting the background value. Abundance and should meet corresponding standards. The selection of the background value can be any point in the 20 scan points before the peak of decafluorotriphenylphosphine (DFTPP), and the background value should be generated by column bleed or instrument background ions.
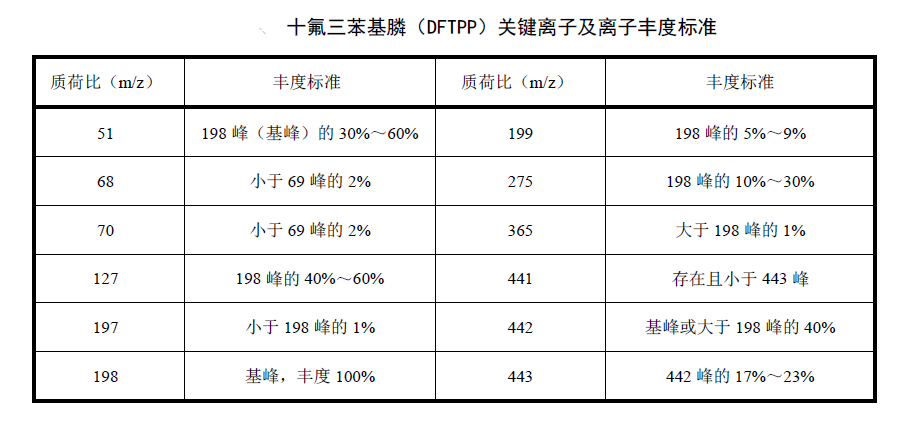
Establish a detection standard curve
Preparation and determination of standard seriesThe standard series of organophosphorus pesticides and substitutes has a total of 6 mass concentration points, which are 0.5ug/ml, 1.0ug/ml, 5.0ug/ml, 10.0ug/ml, 50.0ug/ml and 100ug/ml.
Measure an appropriate amount of the standard stock solution of organophosphorus pesticides and the standard stock solution of the substitute (tributyl deuterated phosphate), dilute to the volume with n-hexane:acetone (1:1), mix well, and prepare 2 mass concentration points. The mass concentrations of the target substance and the substitute are 50.0ug/ml and 100ug/ml in turn; respectively measure an appropriate amount of the standard solution of organophosphorus pesticides and the standard solution of the substitute (tributyl deuterated phosphate), using n-hexane:acetone (1:1) After constant volume, mix well, and prepare 4 mass concentration points. The mass concentrations of the target substance and substitute are 0.5ug/ml, 1.0ug/ml, 5.0ug/ml, and 10.0ug/ml.
Add an appropriate amount of internal standard standard stock solution to the standard series solution to make the internal standard concentration 10.0ug/ml.
Use a micro-syringe to pipette 1.0ul of each of the organophosphorus pesticide column treatment solution and the standard series solution (low concentration to high concentration) and inject them into the gas chromatograph. According to the reference conditions of the instrument, measure and record the retention time of the standard series of targets and the corresponding internal standard, and the peak areas of target ions and auxiliary ions.
Average Relative Response Factor Method
The relative response factor (RRFi) of the target (or surrogate) in the i-th point of the standard curve is calculated according to the following formula.
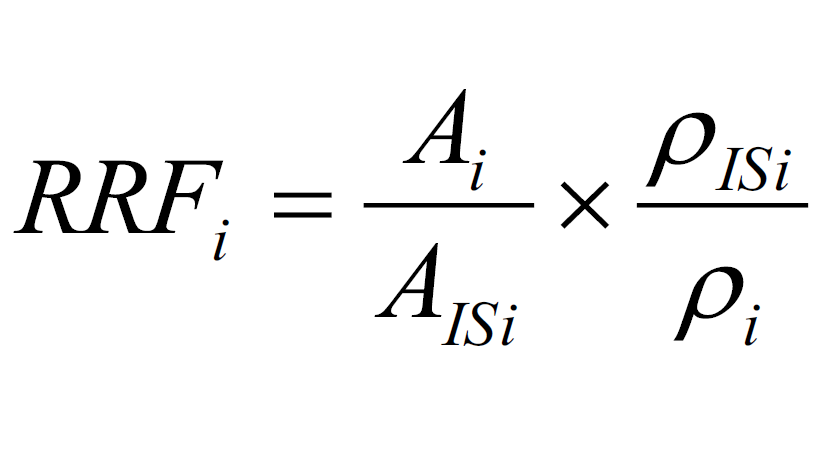
standard curve method
If the relative standard deviation (RSD) of the relative response factor (RRF) of a target in the standard curve is greater than 20%, the target needs to be calibrated with the standard curve. That is, taking the peak area ratio of the target substance and the corresponding internal standard as the ordinate, and the concentration ratio as the abscissa, draw a standard curve, such as the formula y=a+bx.
water samples for testing
Pipette 1.0ul sample with a micro-syringe, inject it into a gas chromatograph, and measure it according to the reference conditions of the instrument. The qualitative analysis and quantitative analysis of the target can be calculated according to the relevant formula.
This method is derived from 《Water Quality Determination of Organophosphorus Pesticides Gas Chromatography Mass Spectrometry》



