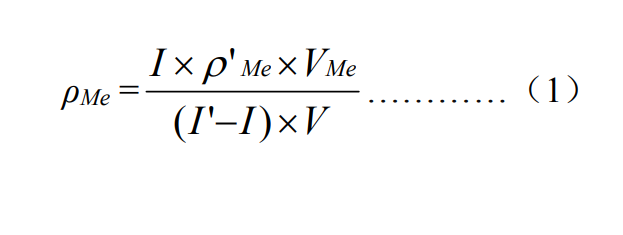The water sample was adjusted to pH 9.0-9.5 with ammonia water-ammonium chloride buffer solution, and dimethylglyoxime reacted with nickel and cobalt in water at a constant pressure of -0.60V on a suspended mercury electrode to form a complex with adsorption. After the enrichment is completed, the electrode potential is scanned from -0.80V to -1.30V, and the nickel and cobalt complexes enriched on the electrode are successively reduced, wherein the reduction potential of nickel is -0.95V, and the reduction potential of cobalt is -1.08. V. The current generated during the reduction process is proportional to the concentration of nickel and cobalt in the sample, and the content of nickel and cobalt is calculated by the standard addition method.

Ways to Eliminate Interference
When the pH value of the water sample after adding ammonia water-ammonium chloride buffer solution is less than 9.0, the cobalt has the superimposed wave interference of zinc near -1.00 V. Adjusting the pH value of the water sample to 9.0-9.5 can eliminate the interference.Water sample pretreatment method
The collected surface water, groundwater, domestic sewage and low-concentration industrial wastewater can be acidified by adding nitric acid to make the pH less than 2, refrigerated and sealed at 4°C, and the storage period is 14 days. After the seawater is collected, it needs to be filtered through a 0.45um membrane and then acidified by adding nitric acid to make the pH less than 2. It is refrigerated and sealed at 4°C for a storage period of 90 days.
Reagents used for testing
1. Nitric acid: 1.42g/ml, excellent grade.2. Ammonia: 0.91g/ml.
3. Ammonia solution: 1+3 (v/v).
Add 10ml of ammonia water to 30ml of laboratory first-grade pure water and mix well.
4. Ammonia chloride solution: ρ(NH4Cl)=54.0g/L.
Weigh 5.4g of ammonium chloride into 100ml of laboratory first-grade pure water and mix well.
5. Butanedione oxime ethanol solution: 0.05%.
Weigh 0.05g of dimethylglyoxime and mix it with 100ml of ethanol, and store in a brown bottle in a dark-tight seal, which can be stored for 9 months.
6. Nickel standard stock solution: 1000mg/L.
Commercially available certified standard solutions can be purchased and stored according to the manufacturer's product instructions.
7. Nickel standard intermediate solution: 10.0mg/L.
Pipette 2.00ml of nickel standard stock solution into a 200ml volumetric flask, add 2.00ml of nitric acid, dilute to the mark with laboratory first-grade pure water, and mix well.
8. Cobalt standard stock solution: 500mg/L.
Commercially available certified standard solutions can be purchased and stored according to the manufacturer's product instructions.
9. Cobalt standard intermediate solution: 2.00mg/L.
Pipette 2.00ml of cobalt standard stock solution into a 500ml volumetric flask, add 5.00ml of nitric acid, dilute to the marked line with laboratory first-grade pure water, and mix well.
10. Nickel and cobalt standard mixed solution: 0.1mg/L, 0.05mg/L
Transfer 2.00ml of nickel standard intermediate solution and 5.00ml of cobalt standard intermediate solution to a 200ml volumetric flask, add 2.00ml of nitric acid, dilute to the marked line with laboratory first-grade pure water, and mix well.
11. Mercury: purity ≥99.999%.
12. Nitrogen: purity ≥99.9%.
13. Membrane: Aqueous membrane with a pore size of 0.45um.
Instruments required for testing water quality
1. Polarograph: with suspended mercury electrode, Ag/AgCl reference electrode and platinum wire auxiliary electrode.2. pH meter: measuring range 0.0-14.0.
3. Micro syringe: 100ul, 1.00ml.
4. Water sample bottle: polyethylene or equivalent material, 100ml, 250ml, 500ml.
5. General laboratory instruments and equipment. Unless otherwise specified, the analysis was performed using a glass measuring instrument conforming to the national standard A.
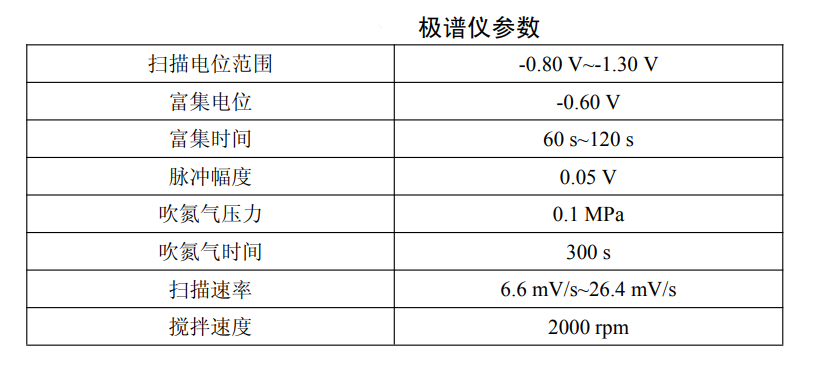
Water sample testing steps
After the water sample returned to room temperature, measure 10.0ml of the water sample in a beaker, add 0.5ml of ammonium chloride solution, adjust the pH of the system between 9.0-9.5 with ammonia solution, and add 0.1ml of dimethylglyoxime ethanol The solution was measured after mixing, and the peak current values of nickel and cobalt were recorded respectively. Then add a certain volume of nickel and cobalt standard mixed solution, measure the peak current value of nickel and cobalt, the peak current value of nickel and cobalt after adding standard should be about 0.5-2 times of the peak current value before adding standard. Finally, the concentration of nickel and cobalt in the water sample was calculated according to the relevant formula used.