The collected and treated water samples are in an acidic medium, and the hydrogen sulfide gas released by the sample is absorbed by the sodium hydroxide solution through on-line heating. Phenylenediamine dihydrochloride reacted to generate methylene blue, and its absorbance was measured at 660nm.

Reagents used for testing
1 Hydrochloric acid: 1.19g/ml.
2 Sodium hydroxide.
3 Ferric chloride.
4N,N-Dimethyl-1,4-phenylenediamine dihydrochloride.
5 Sodium sulfide.
6 Zinc acetate (ZnAc2•2H2O).
7 Sodium carbonate (Na2CO3).
8 Brij35 (Laureth, 30%).
9 Hydrochloric acid solution: VHCl/VH2O=1:9.
10 Color developer:
Pipette 50ml of hydrochloric acid into 800ml of water, add 0.167g of N,N-dimethyl-1,4-phenylenediamine dihydrochloride to dissolve and mix well, make up to 1000ml, add 3ml of Brij35 and mix well. Prepare on the day of use.
11 Ferric chloride solution: 0.005mol/L.
Pipette 50ml of hydrochloric acid into 800ml of water, add 1.33g of ferric chloride and dissolve, dilute to 1000ml, and mix well. Stable for 1 week, store at 4°C when not in use.
12 Sodium hydroxide: 0.10mol/L.
Dissolve 4g of sodium hydroxide in an appropriate amount of water, make up to 1000ml, and mix well.
13 Sodium hydroxide: 0.01mol/L.
Dissolve 0.4g of sodium hydroxide in an appropriate amount of water, dilute to 1000ml, and mix well.
14 Zinc acetate solution: 1.00mol/L.
Dissolve 220g of zinc acetate in 800ml of distilled water, dilute to 1000ml, and mix well.
15 Sodium carbonate solution: 1.00mol/L.
Dissolve 106g of sodium carbonate in 800ml of distilled water, dilute to 1000ml, and mix well.
16 Sulfide stock solution: 100 mg/L.
Dissolve 0.6363g of sodium sulfide in 800ml of sodium hydroxide solution, dilute to 1000ml, mix well, prepare the solution on the day of use, and store in the dark. Or buy certified reference materials.
17 Sulfide use solution: 10mg/L.
Pipette 10.00ml of sulfide stock solution, dilute to 100ml with sodium hydroxide solution, and mix well. The solution was prepared on the day of use and stored in the dark.
18 Nitrogen: purity ≥99.9%
Instruments to be used for testing
1 Continuous flow analyzer: including automatic sampler, chemical reaction module (pretreatment channel, injection pump, reaction channel and flow detection cell, optical path is generally 50mm), peristaltic pump, data processing system.2 Analytical balance: Sensitivity 0.0001g.
3 Ultrasound instrument: frequency 20-40kHz.
4 Sampling bottle: brown narrow bottle, 250ml.
Water sample collection and preservation method
Add 2ml of zinc acetate solution and a few drops of sodium carbonate solution to each liter of water sample to make the pH of the sample ≥ 10. The water sample should be filled with the sample bottle. After sample collection, avoid contact with air, and store at 4°C for no more than 24h in the dark.Detection steps
After the testing instrument is turned on in the specified order, all reagents are replaced with pure water to check the airtightness of the entire analysis flow path and the smoothness of liquid flow. After the baseline is stable, the system starts to pump reagents, and when the baseline is stable again, calibration and blank tests are performed.
Preparation of standard series
Pipette 0ml, 1.00ml, 2.00ml, 4.00ml, 6.00ml, 8.00ml and 10.00ml of standard sulfide solution from a set of 50ml volumetric flasks, dilute them to the marked line with sodium hydroxide solution (6.13) and mix them. Evenly, prepare a standard series of 7 concentration points, the sulfide mass concentration (calculated by S2-) are: 0mg/L, 0.20mg/L, 0.40mg/L, 0.80mg/L, 1.20mg/L, 1.60mg /L, 2.00mg/L.Establishment of standard curve
Measure an appropriate amount of standard series solutions and place them in the sample cup respectively, sample and analyze from low concentration to high concentration, and obtain the signal value (peak height or peak area) of different concentrations of sulfide. Taking the signal value (peak height or peak area) as the ordinate and the corresponding sulfide mass concentration (in S2-, mg/L) as the abscissa, draw a calibration curve.water sample testing
According to the same measurement conditions as drawing the calibration curve, take an appropriate amount of the sample to be tested for measurement, and record the signal value (peak height or peak area). If the concentration is above the peak of the standard curve, dilute the sample. The sulfide concentration in the measured water sample can be calculated according to the corresponding formula.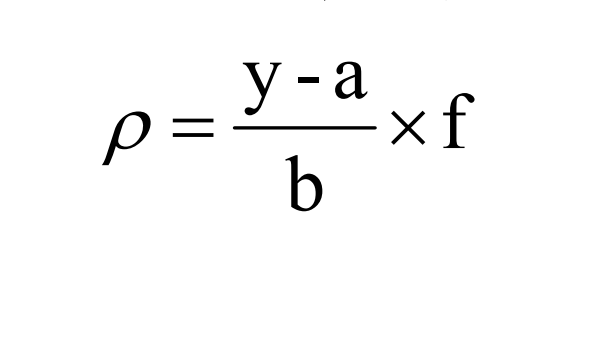
The above content comes from 《DB23T2486-2019 Water Quality Determination of Sulfide Continuous Flow Analysis Method》



