The metal ion indium in water quality can be detected by atomic absorption spectrophotometry. The characteristic spectral lines emitted by cathode lamps or other light sources produce selective absorption, and its absorbance is proportional to the concentration of indium within a certain range.

Common Interfering Substances in Detection
Ag, Al, As, B, Ba, Be, Bi, Cd, Co, Cr, Cu, Mn, Mo, Ni, Pb, Se, Sr, Ti, Tl, V, Zn, K, Na, Mg, Ca, Fe below 500mg/L and Cl- below 10000mg/L had no significant effect on the assay results.
Reagents used for testing
1. Nitric acid 1.42g/ml.2. Palladium nitrate.
3. Magnesium nitrate: excellent grade pure.
4. Indium: Spectrally pure.
5. Nitric acid solution: 50%.
Nitric acid and laboratory grade pure water were mixed in a volume ratio of 1:1.
6. Nitric acid solution: 1%.
Nitric acid and laboratory grade pure water were mixed in a volume ratio of 1:99.
7. Matrix modifier: palladium nitrate-magnesium nitrate mixed solution.
Weigh 0.26 g of palladium nitrate, add 2 ml of nitric acid and a small amount of laboratory first-grade pure water to dissolve. Weigh 0.11g of magnesium nitrate and dissolve it with a small amount of laboratory first-grade pure water. Mix the two solutions and make up to 100ml with laboratory grade water.
8. Indium standard stock solution: 100mg/L.
Accurately weigh 0.1g (accurate to 0.0001g) indium, add 50ml of nitric acid solution, heat to dissolve completely, cool and transfer to a 1000ml volumetric flask, use laboratory first-grade pure water to make up to the mark, and shake well. Transfer to a polyethylene bottle and seal, it can be stored for 2 years. Commercially available certified standard solutions can also be used.
9. Indium standard intermediate solution: 10.0mg/L.
Pipette 10.00ml of indium standard stock solution into a 100ml volumetric flask, dilute to the mark with nitric acid solution, and shake well. Transfer to a polyethylene bottle and seal, it can be stored for 6 months.
10. Indium standard solution: 500ug/L.
Pipette 5.00ml of indium standard intermediate solution into a 100ml volumetric flask, dilute to the mark with nitric acid solution, and shake well. Transfer to a polyethylene bottle and seal, it can be stored for 1 month.
11. Argon: purity ≥99.99%.
12. Filter membrane: 0.45um pore size acetate fiber, polyethylene and other laboratory pure water microporous membrane.
Testing equipment
1. Sample bottle: 500 ml, polyethylene or equivalent.2. Graphite furnace atomic absorption spectrophotometer: with background correction function.
3. Indium hollow cathode lamps or other light sources.
4. Temperature control electric heating plate: with temperature control function.
5. General laboratory instruments and equipment.
Water sample preservation method
After the soluble indium water sample is collected, filter it with a water-based microporous membrane, discard the initial filtrate, collect the required volume of filtrate in a sample bottle, add 1 ml of nitric acid per 100 ml of filtrate to acidify, and measure within 1 month.After the total indium water sample was collected, 1ml of nitric acid was added to each 100ml of the sample for acidification, stored in a sample bottle, and measured within 1 month.
water sample preparation
The water sample of soluble indium can be prepared according to the preservation method. The preparation step of total indium water sample is to measure 50.0ml of water sample mixed evenly in a 150ml glass beaker, add 5ml of nitric acid, place it on a temperature-controlled electric hot plate, cover with a watch glass, and keep it in a slightly boiling state until the sample is uniform and clear Remove the watch glass and evaporate to about 5ml. After cooling, rinse the inner wall of the beaker and the watch glass with water at least 3 times, transfer the whole amount into a 50ml volumetric flask, make up to the mark with water, and shake well for measurement.Detection steps
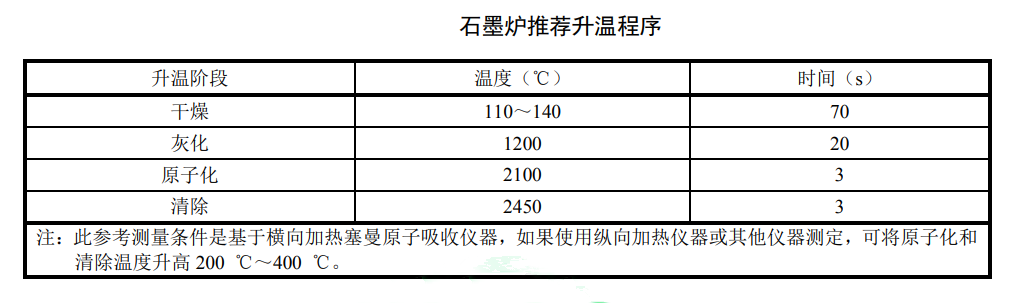
Adjust the instrument to the best working state according to the instrument instruction manual. For the setting conditions of the instrument, please refer to the measurement conditions table. For the recommended heating program of the graphite furnace, see the relevant setting table.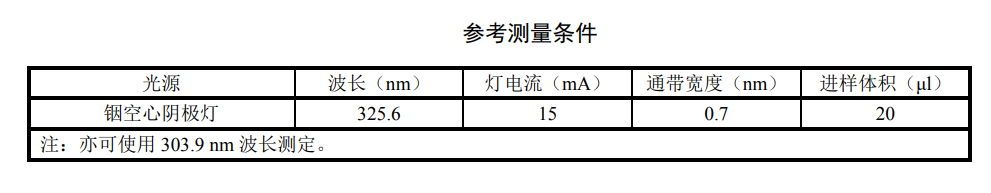
Drawing of the standard curve
Pipette 0.00ml, 0.50ml, 1.00ml, 2.00ml, 3.00ml and 5.00ml of indium standard use solution into a 50ml volumetric flask, dilute to the mark with nitric acid solution, and shake well. The standard series concentrations are 0.00ug/L, 5.00ug/L, 10.0ug/L, 20.0ug/L, 30.0ug/L, 50.0ug/L. According to the reference measurement conditions, 20ul of standard solution and 5ul of matrix modifier were added to the graphite tube from low concentration to high concentration in turn, and the absorbance was measured. Draw a standard curve with the indium concentration as the abscissa and the absorbance as the ordinate.
water sample testing
The prepared water samples were measured according to the same measurement conditions and operation steps as for drawing the standard curve. If the measurement result exceeds the standard curve range, the sample should be diluted with nitric acid solution and re-measured.
The concentration of indium in the water sample can be calculated by the formula ρ=ρ1×D.
The above content comes from 《HJ 1193-2021 Water Quality Determination of Indium Graphite Furnace Atomic Absorption Spectrophotometry》



