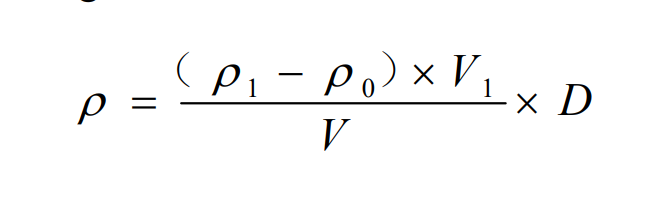After the water sample is filtered or digested, it is sprayed into a lean-burning air-acetylene flame, and the cobalt ground state atoms formed in the high temperature flame selectively absorb the 240.7nm characteristic spectral line emitted by a cobalt hollow cathode lamp or a continuous light source. Its absorbance is proportional to the mass concentration of cobalt within a certain range.

Methods of Eliminating Interference During Detection
Cobalt has spectral interference near the sensitive line 240.7nm, and selecting a narrow spectral passband for measurement can reduce the interference.Hydrochloric acid, phosphoric acid and perchloric acid with concentrations greater than or equal to % have positive interference on the determination of cobalt; sulfuric acid with concentration greater than or equal to 5% has negative interference. After digestion, the concentration of perchloric acid in the sample is controlled below 2% without affecting the determination of cobalt.
When the concentration of Ca is greater than 200mg/L, the concentration of Ni is greater than 40mg/L, and the concentration of Si is greater than 100mg/L, it will cause negative interference to the determination of cobalt.
Detection reagent
1. Nitric acid: 1.42g/ml, excellent grade.2. Perchloric acid: 1.67g/ml, excellent grade.
3. Nitric acid solution: 1+1.
4. Nitric acid solution: 1+99.
5. Cobalt: w≥99.99%, spectrally pure.
6. Lanthanum nitrate or strontium nitrate.
7. Cobalt standard stock solution: 1000mg/L.
Accurately weigh 1g (accurate to 0.0001g) of cobalt, dissolve it in 10ml nitric acid solution, heat to remove nitrogen oxides, cool and transfer to a 1000ml volumetric flask, dilute with water to the mark, and mix well. Transfer it into a polyethylene bottle, seal it, and refrigerate it below 4°C, and it can be stored for 1 year. Commercially available certified standard solutions can also be used.
8. Cobalt standard solution: 50.0mg/L.
Accurately pipette 5.00ml of cobalt standard stock solution into a 100ml volumetric flask, dilute with nitric acid solution to the mark, and mix well. Transfer it into a polyethylene bottle, seal it, and refrigerate it below 4°C, and it can be stored for 30 days.
9. Matrix modifier: lanthanum nitrate solution 20g/L; or strontium nitrate solution, 20g/L.
Weigh 4.7g of lanthanum nitrate or 4.9g of strontium nitrate, dissolve in a beaker with a small amount of water, transfer to a 100ml volumetric flask, dilute with water to the mark, and mix well.
10. Gas: Acetylene, purity ≥99.6%.
11. Supporting gas: air, water, oil and other impurities should be removed before entering the burner.
12. Filter membrane: acetate or polyethylene filter membrane with a pore size of 0.45µm.
13. Quantitative filter paper.
Testing equipment
1. Flame atomic absorption spectrophotometer.2. Light source: cobalt hollow cathode lamp or continuous light source with 240.7nm.
3. Temperature-controlled electric heating plate: The temperature control range is from room temperature to 300 °C, and the temperature control accuracy is ±5 °C.
4. Sample bottle: 500ml, polyethylene or equivalent.
5. General laboratory instruments and equipment.
How to store water samples
Soluble cobalt
Filter the water sample with a filter membrane as soon as possible after collection, discard the initial filtrate, and collect the required volume of filtrate in a sample bottle. Add appropriate amount of nitric acid, acidify to pH≤2, and measure within 14d.total cobalt
Immediately after sample collection, an appropriate amount of nitric acid was added, acidified to pH ≤ 2, and measured within 14 days.water sample preparation
Soluble Cobalt Sample
Take the soluble cobalt sample in a 50ml colorimetric tube to the marking line, add 0.60ml matrix modifier, mix well, and wait for the test.Total cobalt sample
Measure 50.0ml of total cobalt sample into a 250ml glass beaker, add 2.5ml of nitric acid, heat and digest on a temperature-controlled electric heating plate to ensure that the solution does not boil, to about 5ml. Then add 2.5ml nitric acid and 1ml perchloric acid to continue digestion to about 1ml. If necessary, the operation of adding nitric acid and perchloric acid can be repeated until the digestion is complete. After cooling, add 10ml of nitric acid solution, transfer it to a 50ml colorimetric tube (if insoluble residues are present, filter with quantitative filter paper first), use nitric acid solution to make up to the mark, then add 0.60ml of matrix modifier, mix well, and wait for measurement .Preparation of blank samples
The preparation of soluble cobalt and total cobalt laboratory blanks was carried out by following the same procedure as the preparation of the samples, substituting experimental water for the samples.Detection operation steps
Establishment of standard curve
Pipette 0ml, 0.20ml, 1.00ml, 2.00ml, 3.00ml, 4.00ml, 5.00ml of cobalt standard solution into a 50ml colorimetric tube, and use nitric acid solution to dilute to the marking line. The concentration of this standard series is 0mg/ L, 0.20mg/L, 1.00mg/L, 2.00mg/L, 3.00mg/L, 4.00mg/L, 5.00mg/L. Then add 0.60ml of matrix modifier and mix well. According to the measuring conditions of the instrument, measure the absorbance in order from low concentration to high concentration. Taking the mass concentration of cobalt (mg/L) as the abscissa and its corresponding absorbance as the ordinate, a standard curve was established.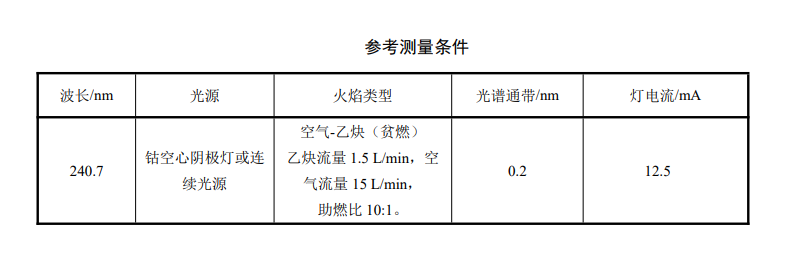
Water sample determination
The measurement of the sample was carried out according to the same instrumental measurement conditions as the establishment of the standard curve. If the measurement result exceeds the range of the standard curve, the sample should be diluted with the zero concentration point solution of the standard series and re-measured. The concentration of cobalt in the water sample was calculated according to the corresponding formula.