Most of the anionic surfactants in reclaimed water come from various industrial wastewaters. In industrial production, it can be used as flocculation and sedimentation, such as wastewater from grain alcohol production, wastewater from papermaking process, wastewater from urban sewage treatment plants, etc. , In addition, it is sometimes used for clarification and purification of drinking water. It can be determined by methylene blue spectrophotometry in routine testing.
The principle is that the cationic dye methylene blue reacts with anionic surfactants in regenerated water, and then generates blue salts, which are extracted with dichloromethane and then measured with a spectrophotometer at a wavelength of 652 nm for absorbance.

Reagents used for testing
1. Sulfuric acid.
2. Methanol.
3. Ethanol: 95%.
4. Dichloromethane.
5. Sulfuric acid solution: 1+35.
6. Sodium hydroxide solution: 40g/L.
7. Formaldehyde solution: 1+99.
8. Hydrochloric acid-ethanol solution: 1+9.
9. Standard stock solution of sodium linear alkyl benzene sulfonate: 1.000g/L.
Weigh (0.100+0.0002) g of sodium straight-chain alkylbenzene sulfonate standard (its alkyl carbon chain is between C10-C13, the average carbon number is 12, and the average relative molecular mass is 344.4), and dissolve it in 50 mL of water. All were transferred to a 100mL volumetric flask, diluted with water to the mark. Shake well and stored in a refrigerator at 4°C. The validity period is two months.
10. Standard solution of sodium linear alkylbenzene sulfonate: 10.0ug/mL.
Pipette 10.00 mL of the standard stock solution of sodium straight-chain alkylbenzene sulfonate and dilute it to 1000 mL with laboratory first-grade pure water. This solution is used now.
11. Methylene blue solution.
Weigh 43.5g of sodium dihydrogen phosphate and dissolve it in 300mL of water. Slowly add 6.8mL of sulfuric acid and transfer it to a 1000mL volumetric flask. Shake well. Weigh 0.03g of methylene blue. Dissolve it with 50mL of water and transfer it to the above volumetric flask. Dilute with water to the mark. Shake well. This solution is stored in a brown bottle.
12. Washing solution: Weigh 43.5g of sodium dioxyphosphate and dissolve it in 300mL of water, slowly add 6.8mL of sulfuric acid. Transfer to a 1000mL volumetric flask, dilute to the mark with water, and shake well.
13. Phenolphthalein indicator solution: 10g/L.
14. Glass wool or absorbent cotton: After extraction with dichloromethane for 4 hours in a Soxhlet extractor, take it out and dry it. Store it in a clear glass bottle.
Testing Equipment
1. Visible light spectrophotometer: equipped with 1cm absorption cell.
2. Separation funnel: 25OmL, with a polytetrafluoroethylene (PTFE) piston.
3. Soxhlet extractor: 150mL flat bottom flask, Φ35X160mm extraction cylinder, serpentine condenser tube.
Sampling Considerations
Sampling and preservation of water samples should use clean glass bottles. Washed with methanol in advance. If the water sample cannot be measured in time, you can save it as follows:
The shelf life is within 24h and should be refrigerated at 4°C;
The shelf life is 1d~4d. Formaldehyde solution should be added,
The storage period is 4d~8d, and dichloromethane should be added to make the dichloromethane in the sample saturated.
Calibration curve drawing steps:
1. Take a set of 9 separatory funnels of 250mL, and transfer them into 0.00mL (blank), 1.00mL, 3.00mL, 5.00mL. Sodium standard solution. Add water to about 100mL, shake well.
2. Add a drop of phenolphthalein indicator solution, dropwise add sodium hydroxide solution until the aqueous solution turns pink, and then dropwise add sulfuric acid solution until the pink color just disappears.
3. Add 25mL methylene blue solution and shake well. Then add 10mL dichloromethane, shake for 30s at a rate of twice per second, let stand for stratification, put the dichloromethane layer into the first pre-filled 50mL washing solution. Two separatory funnels; add dichloromethane to the first separatory funnel to repeat the extraction three times, each 10 mL; combine each dichloromethane into the second separatory funnel with twice per second Shake at a speed for 30s, and let stand for stratification. Pass the dichloromethane layer through glass wool or absorbent cotton and put it into a 50mL volumetric flask; then extract the washing solution with dichloromethane twice (5mL each time). This dichloromethane layer is also Incorporate into volumetric flask, dilute to volume with dichloromethane. Shake well.
4. Take blank as reference. Measure the absorbance of each solution with 1 cm absorbance at 652 nm.
5. Take the measured absorbance as the ordinate and the mass (ug) of the corresponding straight-chain sodium alkyl benzene sulfonate as the abscissa, draw a calibration curve and calculate the normalization equation.
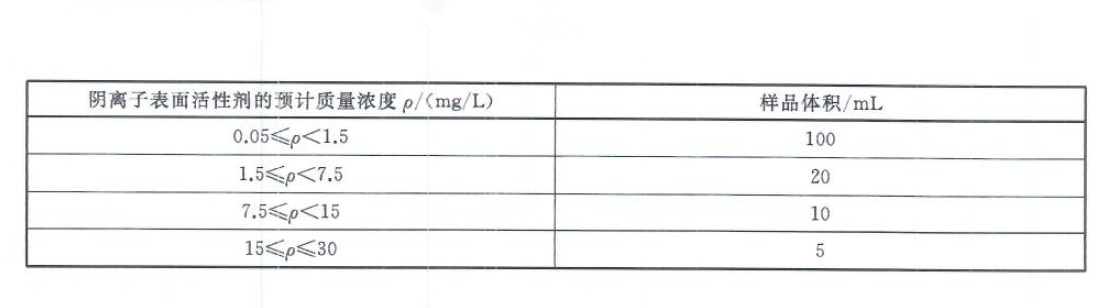
Detection steps
If there is suspended matter in the water sample, the water sample should be filtered through a medium-speed qualitative filter paper before the measurement. Transfer the water sample to a separatory funnel, add water to 100mL, and shake well. Follow steps 2-4 in the calibration curve, find out from the calibration curve or calculate the mass of sodium straight-chain alkylbenzene sulfonate from the calibration curve. If the blue color in the water phase becomes light or disappears, the water sample should be discarded, and the sample volume should be reduced for re-analysis. When measuring low-content water samples, the total amount of dichloromethane used for extraction can be reduced to 25mL, that is, the three extraction volumes are 10mL, 5mL, and 5mL, respectively. Then use 3mL-4mL of dichloromethane to extract the washing liquid. At this time, the lower limit of detection can reach 0.02mg/L.
The content of anionic surfactant in the water sample can be calculated by the corresponding formula.



