The principle of graphite furnace atomic absorption spectrophotometry to detect molybdenum ions or titanium ions in water is to filter or digest the water sample to be tested and inject it into a graphite furnace atomizer. The atom produces selective absorption on the characteristic spectral line 313.3nm or 365.4nm emitted by the molybdenum or titanium hollow cathode lamp, and its absorbance is proportional to the mass concentration of the analyte.

Interference and Elimination Methods in Detection
Ag, Al, As, B, Ba, Be, Bi, Ca, Cd, Co, Cr, Cs, Cu, Fe, Hg, K, Mg, Mn, Na at a concentration of 10 mg/L under the selected assay conditions , Ni, Pb, Sb, Se, Sn, Sr, Tl, Zn have no significant effect on the determination of molybdenum and titanium.
The viscosity, surface tension and composition of samples with different matrices are difficult to match with the standard solution, or the concentration of coexisting ions is high. When it is confirmed that the measurement is affected by the interference inspection, the interference can be eliminated by dilution, matrix modifier or standard addition method.
Reagents required for testing
1. Nitric acid: 1.42g/ml, excellent grade.
2. Nitric acid solution: 1+1, prepared with nitric acid.
3. Nitric acid solution: 0.5% (V/V), prepared with nitric acid.
4. Hydrochloric acid: 1.19g/ml, excellent grade.
5. Hydrochloric acid solution: 1+1, prepared with hydrochloric acid.
6. Hydrogen peroxide solution: Φ(H2O2)=30%.
7. Molybdenum standard solution
Molybdenum standard stock solution: 1000mg/L. Accurately weigh 1.8398g of high-grade pure ammonium molybdate, dissolve it in a small amount of water, transfer it to a 1000ml volumetric flask, and dilute it with water to constant volume. Or use national certified reference materials.
Molybdenum standard intermediate solution: 50.0mg/l.
Accurately pipette 5.00ml of molybdenum standard stock solution into a 100ml volumetric flask, and dilute to volume with nitric acid solution.
Molybdenum standard solution: 500μg/L.
Accurately pipette 1.00ml of molybdenum standard intermediate solution into a 100ml volumetric flask, and dilute to volume with nitric acid solution.
8. Titanium Standard Solution
Titanium standard stock solution: 1000mg/L.
Accurately weigh 1.0000g of titanium (powder or small pieces), add 200ml of hydrochloric acid solution, and heat it to nearly 100°C to dissolve it. Cool, transfer to a 1000ml volumetric flask, dilute with water to volume. Or use national certified reference materials.
Titanium standard intermediate solution: 50.0mg/L.
Accurately pipette 5.00ml of titanium standard stock solution into a 100ml volumetric flask, dilute with nitric acid solution to volume.
Titanium standard solution: 2.50mg/L.
Accurately pipette 5.00ml of titanium standard intermediate solution into a 100ml volumetric flask, and dilute to volume with nitric acid solution.
9. Palladium chloride-magnesium nitrate mixed solution
0.25 g of palladium chloride was weighed, a small amount of water and 1 ml of nitric acid were added, and the mixture was heated to 50° C. to dissolve. Weigh 0.30g of premium pure magnesium nitrate and dissolve it in a small amount of water. Mix the two solutions and dilute to 100ml with water.
Testing Equipment
1. Graphite furnace atomic absorption spectrophotometer.
2. Molybdenum hollow cathode lamp. Titanium hollow cathode lamps.
3. Pyrolytic coated graphite tube.
4. High-purity argon gas cylinder.
5. Temperature-controlled electric heating plate: acid corrosion resistance; can maintain the temperature of 95±5℃.
6. Microwave digestion apparatus: the general power is 600-1500W; the induction temperature reaches ±2.5℃, and the microwave output power is automatically adjusted within 2 seconds after induction; acid-resistant inert plastic material (such as PFA) digestion tank.
7. Filtration device, 0.45um pore size organic filter membrane.
8. Centrifuge: the speed reaches 2000rpm.
9. Sampling bottle: 500ml, made of borosilicate glass, polyethylene or teflon.
10. Laboratory utensils: A-grade glass measuring instruments in line with national standards.
water sample preservation
Soluble metal water samples
Filter the water sample with a filter device as soon as possible after collection, discard 50-100ml of the initial filtrate, wash the sampling bottle with a small amount of filtrate, collect the filtrate in the sampling bottle, and immediately add nitric acid solution to acidify the filtrate to pH 1-2, and measure within 14 days.
Total metal water sample
The water samples were acidified to pH 1-2 with nitric acid solution after collection, and measured within 14 days.
water sample preparation
Total Metal Aqueous Sample Preparation
Hot plate digestion
Measure 50.0ml of the mixed water sample into a borosilicate glass or PTFE beaker. If the organic content of the water sample is low, add 5ml of nitric acid, cover with a watch glass, and heat on a hot plate at 95±5℃ to evaporate To the remaining 5ml, remove and cool, add 3ml of nitric acid, cover with a watch glass, continue to heat and evaporate to near dryness, if necessary, continue to add nitric acid until the digestion is complete (the color of the digestion solution is clear or the appearance no longer changes), cool; if If the organic content of the water sample is high, continue to add 3ml of H2O2 solution, cover with a watch glass, heat and keep the temperature at 95±5℃, until no more bubbles are generated, cool, continue to add H2O2 solution, 1ml each time, cover with a watch glass, and heat And keep the temperature at 95±5℃ until only fine bubbles are generated or the appearance no longer changes, continue to evaporate to near dryness and cool. Add an appropriate amount of nitric acid solution (the final concentration of nitric acid is 0.5% (V/V)) and heat to dissolve the residue. Wash the inner wall of the beaker and the watch glass with a small amount of water, transfer all of them into a 50ml volumetric flask, dilute with water and make up to the mark. Mix well. If there is precipitation, it can be left to stand, or filtered with a 0.45um filter membrane, or centrifuged at 2000-3000rpm for 10min, and the supernatant is taken for testing.
microwave digestion
Measure an appropriate amount of mixed water sample and put it in the microwave digestion tank (the sampling amount + the amount of digestion solvent should not be greater than the volume specified in the digestion tank). If the organic matter content in the sample is low, add 5 ml of nitric acid; if the organic matter content in the sample is high, add 4ml of nitric acid, 1ml of hydrochloric acid and 1ml of hydrogen peroxide solution, placed, cover and seal after the reaction is stable, put into a microwave digestion apparatus, and digest according to the selected heating program (recommended heating program see table). After the digestion is completed, after the temperature in the tank is balanced with the room temperature, the digestion tank is taken out, placed on an electric heating pad, heated and evaporated to near dryness, and cooled. Add an appropriate amount of nitric acid solution (the final concentration of nitric acid is 0.5% (V/V)) and heat to dissolve the residue. Wash the inner wall and lid of the digestion vessel with a small amount of water.
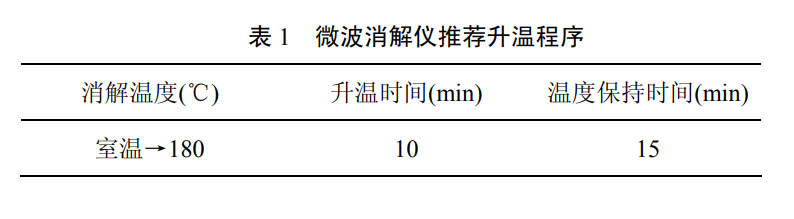
All were transferred into a volumetric flask, diluted with water to the corresponding volume according to the sample concentration, and mixed. If there is precipitation, it can be left to stand, or filtered with a 0.45um filter membrane, or centrifuged at 2000-3000rpm for 10min, and the supernatant is taken for testing.
Soluble metal water samples can be measured directly.
Detection steps
Adjust the instrument to the best working state according to the instrument instruction manual and sample concentration. You can refer to the following figure.
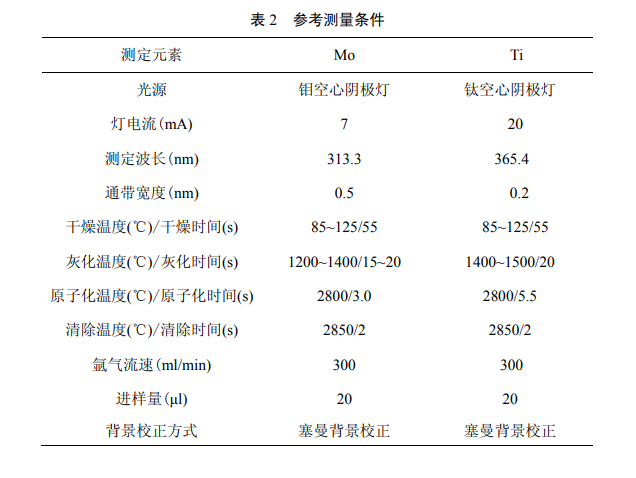
Drawing of Molybdenum Standard Curve
Pipette 0.00, 0.50, 1.00, 2.00, 3.00, 4.00, 5.00ml of the standard use solution of molybdenum into a 50ml volumetric flask, dilute with nitric acid solution to volume. The concentration of standard series is 0.00, 5.00, 10.0, 20.0, 30.0, 40.0, 50.0ug/L, respectively. According to the reference measurement conditions, the absorbance of the standard series is measured sequentially from low concentration to high concentration. Take the absorbance as the ordinate and the mass concentration of the substance to be tested as the abscissa to establish a calibration curve.
Drawing of titanium standard curve
Pipette 0.00, 1.00, 2.00, 3.00, 4.00, 5.00, 6.00ml of titanium standard solution respectively into a 50ml volumetric flask, and dilute to volume with nitric acid solution. The standard series concentrations are 0.00, 50.0, 100, 150, 200, 250, and 300ug/L, respectively. According to the reference measurement conditions, the absorbance of the standard series is measured sequentially from low concentration to high concentration. Take the absorbance as the ordinate and the mass concentration of the substance to be tested as the abscissa to establish a calibration curve.
water sample testing
The prepared water samples were measured and blank absorbance according to the same conditions as the calibration curve was drawn. The mass concentration of the element to be measured in the water sample can be calculated according to the relevant formula.
The above content comes from 《Water Quality Determination of Molybdenum and Titanium Graphite Furnace Atomic Absorption Spectrophotometry》



