Most of the tetraethyl lead in industrial wastewater comes from the petrochemical industry. Its main function is to improve the shock resistance of gasoline, and such compounds are insoluble in water. Therefore, if the oil pollution of gasoline cannot be effectively treated, the tetraethyl lead in it can easily enter the groundwater from the soil or waste water. resulting in serious pollution.
The principle of this method is that under a certain temperature condition, the tetraethyl lead in the water sample in the headspace bottle volatilizes into the headspace. Chromatographic separation and detection by mass spectrometry. Qualitative by comparison with the retention time of the standard substance and the relative abundance of key ions, and quantified by the internal standard method.

Reagents used for testing
1. Methanol: chromatographically pure.2. Standard stock solution of tetraethyl lead: 200mg/L, the solvent is methanol. Commercially available certified standard solution, stored in accordance with the instructions.
3. Tetraethyl lead standard solution I: 2.00mg/L.
Prepare by diluting tetraethyl lead standard stock solution with methanol, transfer to a brown reagent bottle with a teflon-lined screw cap, and store at 0-4°C in the dark for one month.
4. Tetraethyl lead standard solution II: 0.20mg/L.
Prepare by diluting tetraethyl lead intermediate solution with methanol, transfer to a brown reagent bottle with a teflon-lined screw cap, and store at 0-4°C in the dark for one month.
5. Internal standard stock solution: 2000 mg/L, the solvent is methanol.
1,2-Dichlorobenzene-d4 should be selected as the internal standard, and the commercially available certified standard solution can be directly purchased.
6. Internal standard solution: 2.00mg/L.
Prepare by diluting the internal standard stock solution with methanol.
7. Helium, purity ≥99.999%.
Instruments required for testing
1. Gas chromatography-mass spectrometer: The chromatographic part has a split/splitless injection port, and the temperature can be programmed. The mass spectrometer part has an electron impact (EI) ionization source, and has functions such as manual/automatic tuning, data acquisition, quantitative analysis and spectral library retrieval.2. Automatic headspace sampler: The temperature control range is adjustable from room temperature to 100°C.
3. Chromatographic column: Quartz capillary column, 30m×0.25mm, film thickness 0.25mm, the stationary phase is 5% phenyl 95% methyl polysiloxane; or other equivalent capillary column.
4. Sampling bottle: 40ml brown screw-top glass bottle, silicone rubber gasket lined with polytetrafluoroethylene (PTFE), or other similar sampling bottles.
5. Headspace vial: 22ml screw-top or crimp-top headspace vial, sealed with a Teflon silicone rubber gasket.
6. Micro syringe: 5ul, 10ul, 50ul, 100ul and 250ul.
7. General laboratory instruments and equipment.
water sample collection
Add 800ul methanol preservative to the 40ml sampling bottle on site (add 200ul methanol preservative for every 10ml water sample), then collect water samples according to the relevant regulations of volatile organic compounds in HJ/T91 and HJ/T164, and slowly move the water sample along the bottle wall Introduce the sampling bottle until it is full, minimize the escape of tetraethyl lead due to agitation, and avoid introducing air bubbles into the sampling bottle. Parallel double samples were collected on site, one for laboratory determination and one for backup.The water samples should be stored in the dark at about 4°C after collection, and sent to the laboratory as soon as possible, and the analysis will be completed within 24 hours.
Detection steps
Instrument Reference ConditionsHeadspace Autosampler
Heating equilibration temperature: 60°C; heating equilibration time: 10 min; shaking; injection volume: 1.0 ml.
gas color
Injection port temperature: 250°C; carrier gas: helium (4.7); injection mode: split injection (split ratio 5:1); column flow (constant flow mode): 1.0ml/min;
Heating program: 40°C (hold for 1min)→15°C/min 200°C (hold for 1min).
mass spectrometry
Ion source: EI source; ion source temperature: 230°C; ionization energy: 70eV; interface temperature: 280°C; quadrupole temperature: 150°C; scanning method: Selected Ion Scanning (SIM).
Target scan ions: 208, 237, 295; quantitative ions: 237.
Internal standard scan ions: 150, 152, 78; quantitative ions: 150.
Build a working curve
Instrument performance check
Before sample analysis, tuning and checking should be performed according to the calibration compounds and procedures specified in the instrument manual. If the requirements are not met, the parameters of the mass spectrometer should be adjusted or the ion source should be cleaned.
Establishment of working curve
In 5 headspace vials containing 10ml of experimental water, 200ul of methanol were quickly added, the caps were tightly sealed, and 5.0ul of standard use solution II, 2.5ul, 5.0ul, 10.0ul, 25.0ul of standard use solution I prepare standard series with concentrations of 0.10ug/L, 0.50ug/L, 1.00ug/L, 2.00ug/L, 5.00ug/L, and then use it from the septum Add 10.0ul of internal standard to a microsyringe. Then, according to the reference conditions of the instrument, measure sequentially from low concentration to high concentration, and record the calibration series of target compounds, the retention time of the internal standard, and the response value of the quantitative ion.
Under the chromatographic conditions specified in this standard, the total ion current chromatogram of tetraethyl lead is shown in the figure below.
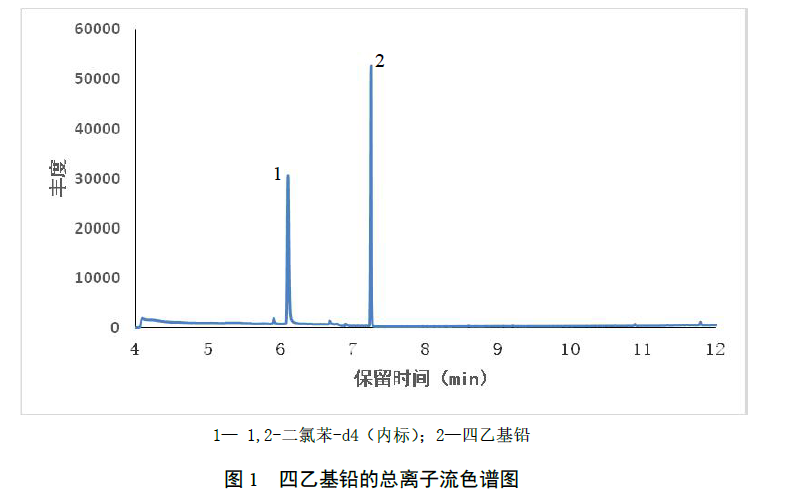
Take the ratio of the concentration of tetraethyl lead to the concentration of the internal standard compound as the abscissa, and take the ratio of the quantitative ion response value of tetraethyl lead to the quantitative ion response value of the internal standard compound as the ordinate to establish the working curve; the average relative response can also be used The factor method is used to calculate.
Water sample determination
Take a 10.0ml water sample into the headspace vial, seal the headspace vial immediately, and add the internal standard with a microsyringe. Measured according to the instrument reference conditions.
Blank test
Take 10.0ml of experimental water in the headspace bottle, quickly add 200ul of methanol, and measure according to the steps and the reference conditions of the instrument.
Qualitative analysis of water samples can be carried out according to the retention time of tetraethyl lead, the relative abundance of key ions in the sample and the relative abundance of key ions in the working curve. The average retention time of tetraethyl lead is obtained by analyzing the calibration solution for many times, and the standard deviation of the average retention time ± 3 times is used as the retention time window, and the retention time of the target substance in the sample should be within the range. The relative abundance deviation of the characteristic ions in the sample mass spectrum and the standard mass spectrum should be within ±30%.
For quantitative analysis, the mass concentration of tetraethyl lead can be directly obtained from the calibration curve, and the concentration of tetraethyl lead in the sample is calculated according to the relevant formula.
The above content comes from "HJ 959-2018 Determination of Tetraethyl Lead in Water Quality by Headspace/Gas Chromatography-Mass Spectrometry"



