Points to note when detecting sodium ions
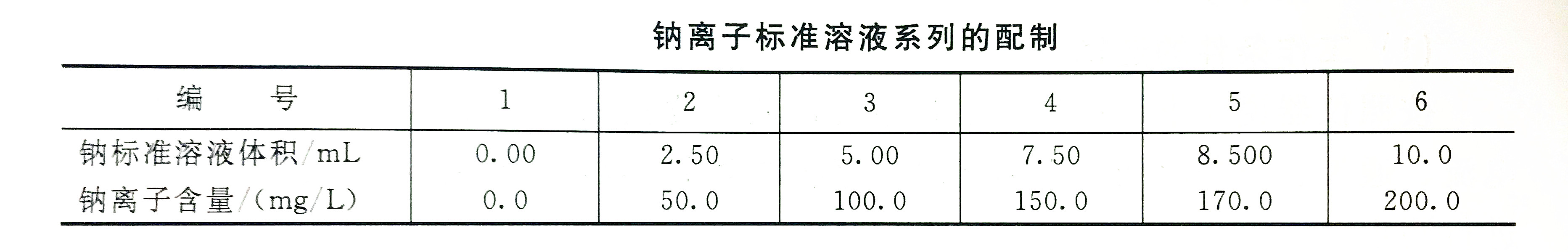
1. Sodium ions will ionize in the air-acetylene flame. To solve this problem, we can add cesium solution to effectively inhibit ionization. In addition, a low-temperature lean flame should be used.2. If the content of sodium ions in the water is greater than 20mg/L, the preparation of the standard series solution needs to be prepared in accordance with the corresponding regulations, and the sodium standard solution is drawn according to the prescribed method and placed in a 50mL volumetric flask, each with 5mL cesium chloride solution , Dilute with water to the mark, shake well. At 330.2nm, adjust the instrument to the best working condition, measure the absorbance one by one from dilute to concentrated, and do a blank experiment at the same time. Using absorbance as the ordinate, the corresponding sodium content (mg/L) as the abscissa, draw a standard curve.
3. SO4, Fe2 below 100mg/L; Zn2+, Cu2 below 20mg/L. Ca2+, C1- below 500mg/L; Mg2+, Si4+ below 40mg/L; PO3 below 60mg/L; 50mg K+ below /L; Al3+ below 30mg/L does not interfere with the measurement. Polyacrylic acid, HEDP, ATMP below 10mg/L; quaternary ammonium salt below 100mg/L and benzotriazole below 3mg/L do not interfere with the determination.
4. When there are more suspended solids in the water sample, it needs to be filtered with medium-speed quantitative filter paper. The filtrate is stored in a polyethylene plastic bottle and can be placed for 2 weeks.
5. The atomic absorption spectrometer used should meet the following indicators
a. Detection limit: In the measurement of circulating cooling water samples, the detection limit of sodium should be less than 0.4mg/L.
b. The working curve is linear: the ratio of the slope in the upper 20% concentration range of the working curve to the slope in the lower 20% concentration range should not be less than 0.7.
c. Minimum precision requirements: the standard deviation of the 10 absorbance of the standard solution with the highest concentration in the working curve should not exceed 1.5% of its average absorbance; the standard of the 10 absorbance of the standard solution with the lowest concentration (not the zero concentration solution) The deviation should not exceed 0.5% of the average absorbance of the highest concentration standard solution.
6. After the measurement is completed, without extinguishing the fire, spray with 1% nitric acid and tertiary reagent water for 5 minutes to clean the sprayer, atomization chamber and burner. After spraying high-concentration acid and alkali water samples, the spray chamber should be thoroughly flushed with water to prevent corrosion. After spraying the organic solution, first spray the organic solvent and acetone for 5 minutes, then spray 1% nitric acid and tertiary reagent water for 5 minutes each.
Pay attention to keeping the combustion head clean, especially after measuring high salt content water, there will be a phenomenon of clogging of the slit, the light green burning layer of the flame close to the slit mouth has a partial sawtooth shape, and intermittent red flashes or red flames appear in the flame in severe cases. . Therefore, it is necessary to frequently scrub the slits with cotton dipped in alcohol, and use paper or cork to remove the deposits in the slits and on both sides of the slits under the condition of passing air to ensure the normal flame. When cleaning the atomizer, the water, electricity, and gas must be turned off.
The above are the issues that everyone needs to pay attention to when detecting sodium ions



