Iron is a relatively common heavy metal element in water. If proper iron is contained in drinking water, it is beneficial to the human body. But once the iron element in the water exceeds the standard for long-term drinking, it will cause harm to the human body. Therefore, the relevant departments have made clear regulations on the iron content in the water. The iron element in water mainly comes from minerals and oxides in the earth's crust. Generally, iron in water exists in the form of divalent, trivalent, and iron oxide. I have introduced you how to detect divalent iron in water before, and today we will talk about how to use sulfosalicylic acid spectrophotometry to detect the total iron content in water.

Instruments and reagents used for testing
1. Spectrophotometer
2. Analytical balance
3. Dry box
4. 50mL colorimetric tube with stopper
5. Concentrated hydrochloric acid (excellent grade pure)
6.1mol/L hydrochloric acid solution
7.30% sulfosalicylic acid solution
8. Ammonium persulfate
9. Iron standard solution
a. Stock solution
Weigh 0.1000g pure iron wire, add 50mL hydrochloric acid solution (1mol/L) and heat it to dissolve completely, add a small amount of ammonium persulfate, boil for a few minutes, transfer it into a 1L volumetric flask, and dilute to the mark with first-grade reagent water. Or weigh 0.8634g ferric ammonium sulfate, dissolve it in 50mL hydrochloric acid solution, transfer it to a 1L volumetric flask after it is completely dissolved, dilute to the mark with first-grade reagent water, and calibrate its concentration by gravimetric method.
b. Working solution
Take 100mL of the above stock solution and pour it into a 1L volumetric flask, add 50mL of hydrochloric acid solution, and dilute to the mark with first-grade reagent water.
Detection steps of total iron content in water
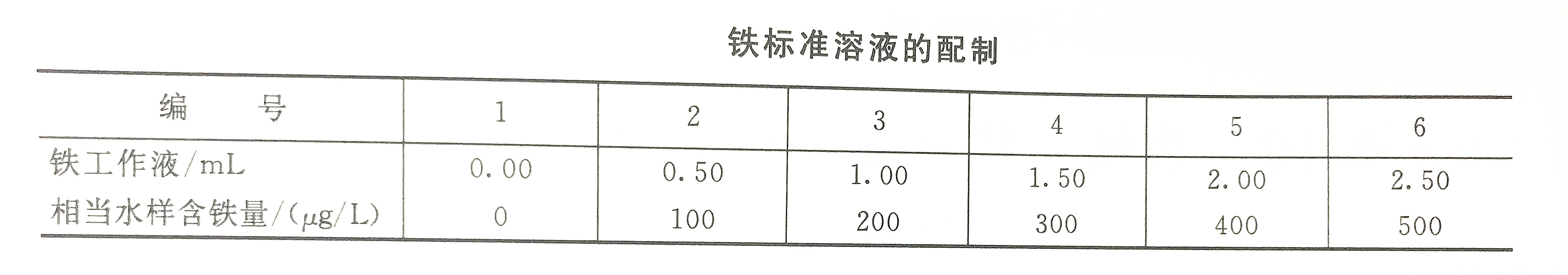
1. Draw a working curve
According to the preparation table of iron standard solution, take a set of iron working solution and inject it into a set of 50mL colorimetric tubes, add 1m concentrated acid salt respectively, and dilute to about 40mL with first-grade reagent water. Add 4mL sulfosalicylic acid solution, shake up, add about 4mL of concentrated ammonia water, shake up to make the pH reach 9-11, and dilute to the mark with first-grade reagent water. After mixing, use a spectrophotometer to measure the absorbance with the first-grade reagent water as a reference. Draw a working curve of the measured absorbance and the corresponding iron content.
2. Water sample determination
a. Wash the sampling bottle with hydrochloric acid solution (1+1), then wash it three times with first-grade reagent water, and then add concentrated hydrochloric acid (2mL of concentrated hydrochloric acid per 500mL water sample) to the sampling bottle for direct sampling.
b. Take 50mL of water sample into a 100~150mL beaker, add 1mL of concentrated hydrochloric acid, boil and concentrate to about 20mL, and then transfer to the colorimetric tube after cooling, and clean the beaker 2~3 times with a small amount of first-grade reagent water, washing liquid one And injected into the colorimetric tube, but its total volume should not be greater than 40mL. Perform color development according to the steps of drawing a working curve, and measure the absorbance on a spectrophotometer. According to the measured absorbance, check the working curve to get the iron content in the water sample.
Precautions during testing
1. When the iron content of the water sample is less than 50ug/L, the o-phenanthroline method should be used for the determination. When the iron content is greater than 500ug/L, the water sample can be diluted as appropriate and measured.
2. For colored water samples, the amount of ammonium persulfate added should be increased, and the iron content of ammonium persulfate should be deducted through a blank test. Ammonium persulfate can also be used as a solution, but because its solution is unstable, it should be prepared during use.
3. In order to ensure normal color rendering, attention should be paid to whether the ammonia concentration is reliable.
4. In order to ensure that the water sample is not contaminated, before using glassware such as sampling bottles, beakers, colorimetric tubes, etc., use hydrochloric acid (1+1) to boil and wash.
5. Phosphate has no interference to this method.
6. After the water sample is filtered, the measurement result is low.



