Iodometric method is suitable for daily water and wastewater with sulfide content above 0.40mg/L. The principle is that sulfide reacts with excess iodine under acidic conditions, and the remaining iodine is titrated with sodium thiosulfate, which is determined by sodium thiosulfate. The amount of solution consumed indirectly calculates the sulfide content.

Reagents and equipment used
1. Acidification-blowing-absorption device2. Constant temperature water bath
3. Iodine measuring flask 150mL or 250mL
4. Burette brown, 25mL or 50mL
5. Hydrochloric acid
6. Phosphoric acid
7. Acetic acid
8. High purity nitrogen
9. Hydrochloric acid solution
10. Phosphoric acid solution
11. Acetic acid solution
12. Sodium hydroxide solution
Dissolve 40g sodium hydroxide in 500mL tertiary reagent water, cool to room temperature, and dilute to 1000mL.
13. Acetic acid solution
Weigh 220g zinc acetate, dissolve it in tertiary reagent water and dilute to 1L.
14. Potassium dichromate standard solution
Weigh 4.9030g of standard or premium grade pure potassium dichromate solution that has been dried at 105°C for 2 hours and dilute to 1000mL.
15. 1% starch indicator
Weigh 1g of soluble starch and use a small amount of tertiary reagent water to make a paste, and then dilute it to 100 mL with just boiling tertiary reagent water.
16. Potassium iodide
17. Sodium thiosulfate standard solution
a. Preparation Weigh 24.5g pentahydrate sodium thiosulfate and 0.2g anhydrous sodium carbonate dissolved in the third-grade reagent water, transfer to a 1000mL brown volumetric flask, dilute to the mark and shake.
b. Calibrate in a 250mL iodine flask, add 1g potassium iodide and 50mL tertiary reagent water, add 15.00mL potassium dichromate standard solution, add 5mL hydrochloric acid solution, close the plug and mix, keep it in the dark for 5 minutes, and use it to be calibrated When the sodium thiosulfate solution is titrated until the solution is light yellow, add 1mL starch indicator, continue titration until the blue color just disappears, record the standard solution dosage, and make a blank titration.
18. Sodium thiosulfate standard solution
Pipette 10mL just calibrated sodium thiosulfate standard solution in a 100mL brown volumetric flask, dilute to the mark with tertiary reagent water, shake well, and prepare it before use.
19. Iodine Standard Stock Solution
Pipette 12.7g of iodine in a 500mL beaker, add 40g of potassium iodide, add an appropriate amount of tertiary reagent water to dissolve, transfer to a 1000mL brown volumetric flask, dilute to the mark, and shake.
20. Iodine Standard Solution
Pipette 10.0mL iodine standard stock solution in a 100mL brown volumetric flask, dilute to the mark with tertiary reagent water, shake well, and prepare before use.
Sampling and storage when detecting sulfide in water by iodometry
When sampling, first add a certain amount of zinc acetate solution to the sampling bottle, then add water sample, and then add an appropriate amount of sodium hydroxide solution dropwise to make it alkaline and generate zinc sulfide precipitation. Under normal circumstances, add 0.3mL zinc acetate solution (1mol/L) and 0.6mL sodium hydroxide solution (1mol/L) per 100mL water sample to make the pH value of the water sample 10-12. When encountering alkaline water samples, carefully add acetic acid solution (1+1) to neutral before proceeding as above. When the sulfide content is high, more fixatives can be added as appropriate until the precipitation is complete. After the water sample is full, it should be tightly sealed and stored, taking care not to leave bubbles, and then inverted and mixed thoroughly to fix the sulfide. Samples should be analyzed immediately after collection, otherwise they should be stored at 4°C under light protection and analyzed as soon as possible.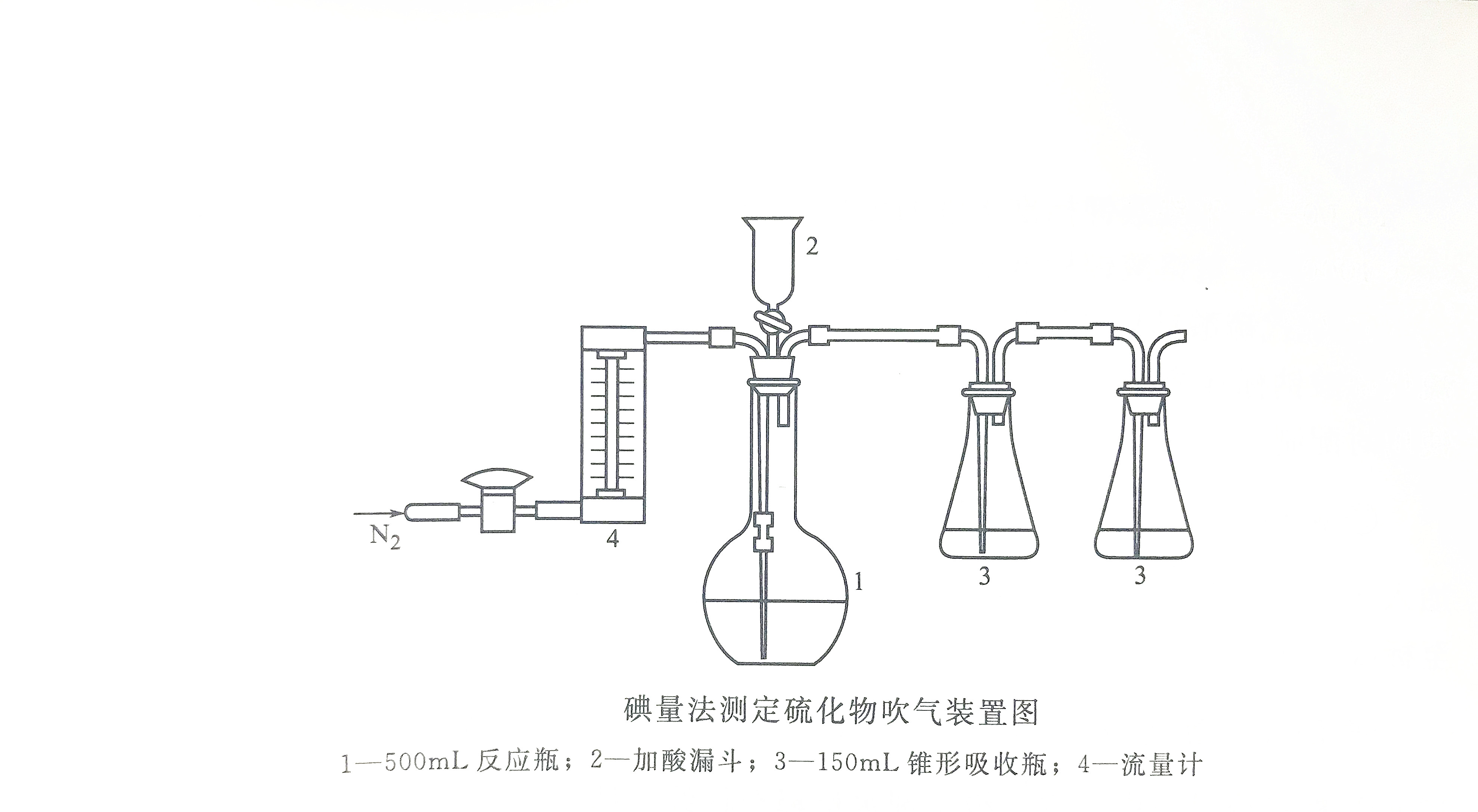
Water quality test operation steps
1. Pretreatment of water samples
① Connect the acid blowing-absorption device according to the steps, and check the air tightness of each part through the carrier gas.② Dilute 2.5mL zinc acetate solution in two absorption flasks and dilute to 50mL with tertiary reagent water.
③Take 200 mL of the water sample that has been fixed and mixed on site into the reaction flask, put it in a constant temperature water bath, and install the air duct, acid addition funnel and absorption bottle. Turn on the air source, blow nitrogen continuously at a flow rate of 400 mL/min for 5 minutes to drive out the air in the device, and turn off the air source.
④ Add 20 mL of phosphoric acid solution (1 10 1) to the acid addition funnel, and close the piston quickly after the phosphoric acid is nearly all flowing into the reaction flask.
⑤Turn on the air source, and when the temperature of the water bath is controlled at 60~70℃, blow for 20min at a flow rate of 75~100mL/min, blow for 10min at a flow rate of 300mL/min, and then blow for 5min at a flow rate of 400mL/min to remove the last residue. Hydrogen sulfide gas in the device. Turn off the gas source, and determine the sulfide content in the two absorption bottles according to the following iodometric operation steps.
2. Measurement procedure
Add 10.0mL iodine standard solution (0.01mol/L) to each of the two samples prepared by pretreatment, and then add 5mL hydrochloric acid solution (1+1), and mix tightly. Place in a dark place for 10 minutes, titrate with sodium thiosulfate standard solution (0.01mol/L) until the solution is light yellow, add 1mL starch indicator, and continue titration until the blue just disappears.Use tertiary reagent water instead of the water sample, add the same volume of reagent as the measurement, and perform a blank test according to the steps of sample pretreatment and sample measurement. Finally calculate the sulfide content.



