1. Problems that need to be paid attention to when collecting water samples
The water samples should be collected in glass bottles or polyethylene bottles. The measurement should be carried out immediately after sampling. If the detection cannot be performed directly due to conditions, the measurement personnel need to add an appropriate amount of sulfuric acid to the water sample to adjust the pH of the water sample to less than Store at 4 degrees Celsius at 2 o'clock. After the water sample is allowed to stand for 30 minutes, use a pipette to remove the water sample one or more times. The water inlet tip of the pipette should be inserted below 50mm from the surface of the water sample, and then a preservative should be added for storage.

2. Matters needing attention when testing
If the mercury salt is digested to form a mercury ammonium complex during detection, you can add an appropriate amount of sodium thiosulfate to decompose the complex during alkaline distillation. Before distillation, carefully check the connection of the distillation device, and no gas leakage, otherwise the measurement result will be affected due to the loss of the distilled gas. Avoid bumping during distillation, otherwise the measurement result will be low due to incomplete absorption. The solution in the distillation flask must be kept alkaline during distillation. If the bottle is still clear and transparent during the distillation, check the acidity and alkalinity of the solution after the distillation. If necessary, add appropriate amount of water and sodium hydroxide solution to distill again. For difficult-to-digest organic nitrogen compounds, the digestion time can be appropriately increased, or mercury sulfate solution can be added as a catalyst, and 2 mL per water sample can be added.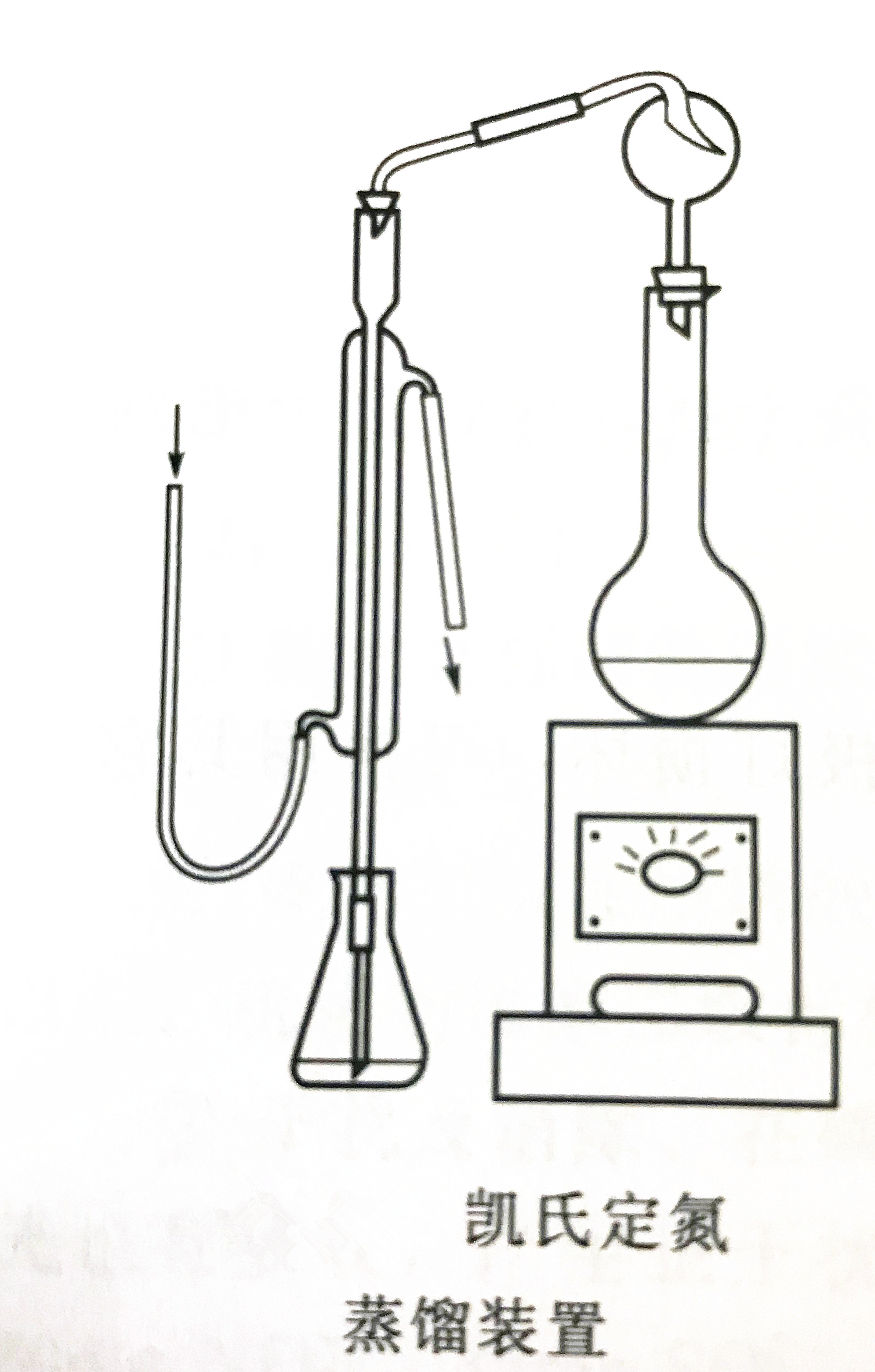
3. Other issues that need attention
Kjeldahl nitrogen refers to the nitrogen content measured by the Kjeldahl method. Including ammonia nitrogen and organic nitrogen compounds that can be converted into ammonium salts under these conditions. Such organic nitrogen compounds are mainly amino acids, proteins, peptones, urea, nucleic acids and synthetic organic nitrogen compounds with negative trivalent nitrogen. Therefore, you should use this method according to your specific needs when testing.After distillation, the residual liquid contains mercury salt precipitation, which should be filtered and separated for proper disposal. Treatment of waste liquid containing mercury salts: control the acidity of the solution to 0.3moL/L, and add excess sulfide to produce mercury sulfide precipitation.



