Coexisting ions and compounds in groundwater and surface water do not interfere with the determination at common concentrations. When the concentration of calcium is higher than 1000mg/L, the absorption of cadmium is inhibited. When the concentration is 2000mg/L, the signal is inhibited by 19%. Under weak acid conditions, when the content of hexavalent chromium in the sample exceeds 30mg/L, due to the formation of chromium The precipitation of lead acid makes the measurement result of lead low. In this case, 1% ascorbic acid needs to be added to reduce hexavalent chromium to trivalent chromium. When the content of soluble silicon in the sample exceeds 20mg/L, it interferes with the determination of zinc, which makes the measurement result low. Adding 200mg/L calcium can eliminate this interference. When the iron content exceeds 100mg/L, the absorption of zinc is inhibited. When the salt content in the sample is very high and the analysis wavelength is below 350nm, non-characteristic absorption may appear. For example, high concentration of calcium, due to non-characteristic absorption, that is, background absorption, makes the determination result of lead higher.
For the above reasons, it is necessary to check whether there is matrix interference or background absorption before analyzing samples. Generally, the degree of matrix interference is judged by measuring the recovery rate of standard addition. By measuring the absorption at a non-characteristic absorption line within 1nm near the analysis line, the size of the background absorption can be judged. The non-characteristic absorption line corresponding to the selected analysis line can be selected according to the background correction near line wavelength table.
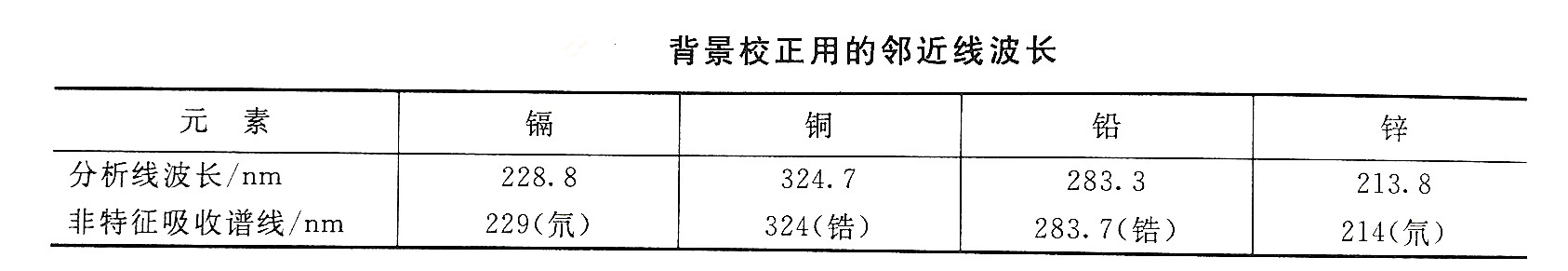
According to the results of the test, if there is matrix interference, interference inhibitors can be added, or the standard addition method can be used to determine and calculate the results. If there is background absorption, use automatic background correction device or adjacent non-characteristic absorption line method to correct. The latter method is to subtract the absorption value near the non-characteristic absorption line from the absorption value measured at the analysis line to obtain the true absorption of the measured element atom. In addition, chelating extraction or sample dilution can also be used to separate or reduce components that produce matrix interference or background absorption.
When using APDC-MIBK extraction system, if the chemical oxygen demand of the sample is greater than 500mg/L, it may affect the extraction efficiency. When the iron content is less than 5mg/L, it will not interfere with the determination. If the amount of iron in the water sample is high, you can use the potassium iodide-methyl isobutyl ketone (KI-MIBK) extraction system for better results. If a certain type of complexing agent in the sample forms a complex with the measured metal ion, which is more stable than the complex formed with ammonium pyrrolidine dithiocarbamate or potassium iodide, it must be oxidized and decomposed before the measurement.



