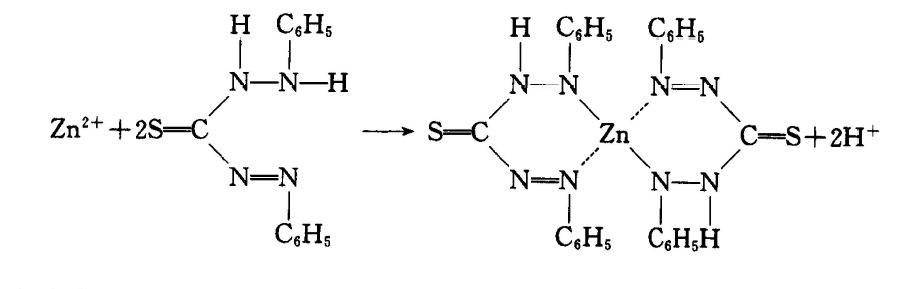
The content of total zinc in sewage was detected by dithizone spectrophotometry. The principle is that in the acetate buffer medium with pH value of 4.0-5.5, zinc ions in water and dithizone form red chelate. After extraction with carbon tetrachloride, colorimetric determination can be performed at a wavelength of 535 mm. The determination range of this method is 0.005mg/L~0.05mg/L. If the content of zinc is not within the range of determination, the sample can be diluted or concentrated appropriately.
Reagents and instruments used for testing
Detection reagent
1. Hydrochloric acid 1.19g/mL excellent grade pure
2. Nitric acid 1.40g/mL, excellent grade pure
3. Perchloric acid 1.67g/mL, excellent grade pure
4. Glacial acetic acid
5. Ammonia 0.90g/mL, excellent grade pure
6. Carbon tetrachloride
7. Hydrochloric acid: 2mol/L
Take 100mL of hydrochloric acid and dilute it to 600mL with first-grade pure water.
8. Nitric acid solution (1+9)
Slowly add 100 mL of nitric acid to 900 mL of first-grade pure water.
9. Nitric acid solution (0.2+99.8)
Slowly add 2 mL of nitric acid to 998 mL of first-grade pure water.
10. Sodium acetate buffer solution
Dissolve 68 g of sodium acetate trihydrate in first-grade pure water and dilute to 250 mL, prepare 250 mL of 12.5% (V/V) acetic acid solution, mix the above two solutions in equal volumes and place them in a separatory funnel. The hydrazone stock solution was extracted several times until the extract was green and then extracted with carbon tetrachloride to remove excess dithizone.
11. Sodium Thiosulfate Solution
Dissolve 25g of sodium thiosulfate in water and dilute to 100mL, extract with 10mL of dithizone carbon tetrachloride solution each time, until the dithizone solution turns green, then extract with carbon tetrachloride to remove excess disulfide hydrazone.
12. Refined dithizone
Weigh 0.5 g of dithizone and dissolve it in 100 mL of chloroform, place the obtained solution in a separatory funnel, add 50 mL of 1% (V/V) ammonia water and shake it, put it into the water phase, repeat four times, and combine the obtained water phases , filter with a funnel with a small group of absorbent cotton inserted into the neck of the tube to remove residual chloroform, and then use hydrochloric acid or sulfuric acid dioxide (it is appropriate to use sulfuric acid dioxide, sulfur dioxide has a reducing effect, and will not introduce traces of the solution into the solution. Metal). The precipitated dithizone was extracted several times with chloroform (15mL-20mL each time), combined the extracts, washed with water several times, evaporated the chloroform on a water bath at 50°C, dried in a desiccator, and stored. Reserve in the dark.
13. Dithizone Stock Solution
Dissolve 125 mg of purified dithizone in 500 mL of carbon tetrachloride, and store it in a brown reagent bottle in a refrigerator.
14. Dithizone solution 0.01%
Take 40mL of dithizone stock solution, dilute it with carbon tetrachloride to 100mL, and prepare it when it is used.
14. Dithizone solution 0.001%
15. Take 10mL of dithizone solution, dilute it with carbon tetrachloride to 100mL, and prepare it as needed.
16. Sodium citrate solution
Weigh 10g of sodium citrate dihydrate and dissolve it in 90mL of water, first extract it several times with dithizone stock solution until the extract turns green, then extract with carbon tetrachloride to remove excess dithizone, this reagent is used for glass Final wash of utensils.
17. Zinc stock solution 100mg/L
Accurately weigh (0.1000 ± 0.0001) g zinc particles (purity 99.9%) or weigh (0.124 5 ± 0.0001) g zinc oxide (spectrally pure), dissolve in 5 mL of hydrochloric acid, transfer to a 1000 mL volumetric flask, and dilute with water to the marked line .
18. Zinc standard solution 1.0mg/L
Take 10mL of zinc stock solution in a 1000mL volumetric flask and dilute with water to the mark.
Instruments used for testing
1. Spectrophotometer
2. Separation funnel 125mL
3. Glassware
Specific detection steps
1. Digestion first
Take an appropriate amount of sample, add 5 mL of nitric acid, heat and evaporate to about 10 mL on a hot plate, remove and cool, add 5 mL of nitric acid and 4 mL of perchloric acid (when the sample is not seriously polluted, a small amount of hydrogen peroxide can be used instead of perchloric acid), continue Heat digestion, evaporate to near dryness, dissolve with hot nitric acid solution, filter into a 100mL volumetric flask, and wash with hot water, after cooling, adjust the pH value to 2-3 with hydrochloric acid or ammonia water, and finally dilute with water to the mark.
2. Extraction color
Take 10mL of digestion solution and put it in a 125mL separatory funnel, add 5mL of sodium acetate buffer solution and 1mL of sodium thiosulfate solution, shake well, then add 10mL of dithizone stock solution, shake for 4min, after layering, put it in the separatory funnel A small ball of absorbent cotton was inserted into the neck of the tube, and the dithizone carbon tetrachloride layer was placed in a 20mm cuvette.
3. Absorbance measurement
Using carbon tetrachloride as a reference, the extract was placed at a wavelength of 535 nm to measure the absorbance.
4. Determining the Zinc Content
Subtract the absorbance of the blank test from the absorbance of the sample, and check the zinc content from the working line.
Draw working curve
Add zinc standard solution 0mL, 0.50mL, 1.00mL, 2.00mL, 3.00mL, 4.00mL, 5.00mL to the 125mL separatory funnel respectively, add water to 10mL, measure the absorbance according to the detection steps, and draw the absorbance (absorbance value of the standard solution). Subtract zero standard absorbance) versus the amount of zinc. The working curve needs to be redrawn for each batch of samples analyzed.
Blank test
Take the same amount of laboratory ultrapure water as the water sample, operate according to the detection steps, and use the obtained absorbance to get the blank value from the working curve. If the blank value exceeds the confidence interval, the reason should be checked.
Finally, the content of zinc in the water sample was calculated according to the corresponding formula.



