Pyridine is estimated that many people do not understand that it is an organic compound that mainly exists in petroleum, coal tar and other substances. Generally, it is rarely encountered in the environment, but as a raw material for the production of drugs, dyes and other substances, it is often used in many chemical industries. Therefore, most of the pyridine pollution in the environment is caused by the leakage of production links and the illegal discharge of industrial wastewater.
Generally, the content of pyridine in surface water can be detected by gas chromatography. When the sampling volume is 1000ml and the constant volume of the sample is 1.0ml, the detection limit of the method for 12 nitrophenolic compounds is 0.2ug/L-2ug/L , the lower limit of determination is 0.8ug/L-8ug/L.

Reagents required for testing
1. Sulfuric acid 1.84g/ml, excellent grade pure.
2. Sodium hydroxide.
3. Sodium chloride: excellent grade pure.
Burn at 500℃-550℃ for 2h, cool to room temperature, and store in a desiccator for later use.
4. Sodium thiosulfate.
5. Methanol: chromatographically pure.
6. Pyridine: purity ≥99.5%.
7. Sulfuric acid solution: 1+1.
8. Sodium hydroxide solution 10mol/L.
9. Standard stock solution 1000mg/L.
First add an appropriate amount of methanol to a 100ml volumetric flask, then weigh 100mg (accurate to 0.1mg) of pyridine into the volumetric flask, make up to the mark with methanol, and mix well. Refrigerate below 4°C, keep it in a sealed place away from light, it can be stored for 3 months, and return to room temperature before use. Or directly purchase certified standard solutions.
10. Standard use solution 10.0mg/L.
Dilute the standard stock solution with water and prepare it for immediate use.
11. Carrier gas: high-purity nitrogen, purity ≥99.999%.
12. Combustion gas: high-purity hydrogen, purity ≥99.999%.
13. Supporting gas: air, dehydrated by silica gel, dehydrated by activated carbon.
Instruments that need to be prepared for testing
1. Sampling bottle: 40ml brown screw-top glass bottle with a silicone rubber-PTFE liner screw cap.
2. Gas chromatograph: with split/splitless inlet and hydrogen flame ionization detector (FID), chromatography data processing workstation.
3. Chromatographic column I: The specification is 30m (column length) × 0.32mm (inner diameter) × 0.5um (film thickness), 100% polyethylene glycol stationary phase capillary column, or other equivalent capillary column.
4. Chromatographic column II: 30m (column length) × 0.25mm (inner diameter) × 1.4um (film thickness), 6% nitrile propylphenyl + 94% dimethylpolysiloxane stationary phase capillary column, or other Equivalent capillary column.
5. Automatic headspace sampler: The temperature control accuracy is ±1℃.
6. Headspace vials: headspace vials (22ml), polytetrafluoroethylene (PTFE)/siloxane seals, caps (screw caps or single-use glands), can also be used with automatic headspace samplers Matching glass headspace vials.
7. Pipette: 1ml~10ml.
8. Analytical balance: accuracy 0.1 mg.
Collection and preservation of water samples
Be sure to check the pH value of the water sample before sampling, and adjust the pH to 6-8 with sulfuric acid solution or sodium hydroxide solution according to the pH value measurement result. When collecting the water sample, the water sample should overflow in the water sample bottle without leaving a head space. When sampling, try to avoid or reduce the exposure of water samples to the air. All water samples were collected in parallel. If there is residual chlorine in the water sample, add 100 mg of sodium thiosulfate per 1000 ml of water sample.According to the same steps as the water sample collection, put a piece of experimental water into a water sample bottle for sealing in the laboratory, bring it to the sampling site, and transport it back to the laboratory together with the water sample for storage.
After the water sample is transported back to the laboratory, it should be tested as soon as possible. If it cannot be detected in time, it should be refrigerated below 4°C, protected from light and sealed, and the detection should be completed within 3 days. The area where the water sample is stored should be free of volatile organic compounds, and the water sample should be returned to room temperature before the measurement.

Test sample preparation method
Add 3g of sodium chloride to the prepared headspace bottle in advance, then add 10.0ml of water sample, seal it immediately, shake well, and wait for the test; laboratory blank samples and full-program blank samples can be carried out according to this method preparation.
Check the specific steps
Instrument Setup Reference Conditions
1. Headspace sampler reference conditions
Heating equilibrium temperature: 70°C; heating equilibrium time: 30min; injection valve temperature: 100°C; transfer line temperature: 110°C; injection volume: 1.0ml; pressure equilibrium time: 1min; injection time: 0.2min.
2. Gas chromatograph reference conditions
Injection port temperature: 200°C; detector temperature: 230°C; column temperature: 70°C; column flow rate: 3.0ml/min; combustion gas (high-purity hydrogen) flow rate: 40ml/min; air) flow rate: 350ml/min; makeup gas flow rate: 30ml/min; split ratio of 10:1.
draw standard curve
Add 3g of sodium chloride to 5 headspace bottles in advance, accurately add 9.80ml, 9.50ml, 9.00ml, 8.00ml and 5.00ml of water in sequence, and then add 0.20ml, 0.50ml, 1.00ml, 2.00ml in sequence and 5.00ml of pyridine standard solution, prepared into standard series with pyridine mass concentrations of 0.20mg/L, 0.50mg/L, 1.00mg/L, 2.00mg/L and 5.00mg/L, immediately cover and seal, and mix well . According to the reference conditions of the instrument, the samples were sequentially injected and analyzed from low concentration to high concentration, and the retention time and response value of pyridine were recorded. Taking the pyridine concentration as the abscissa and its corresponding response value as the ordinate, a standard curve was established.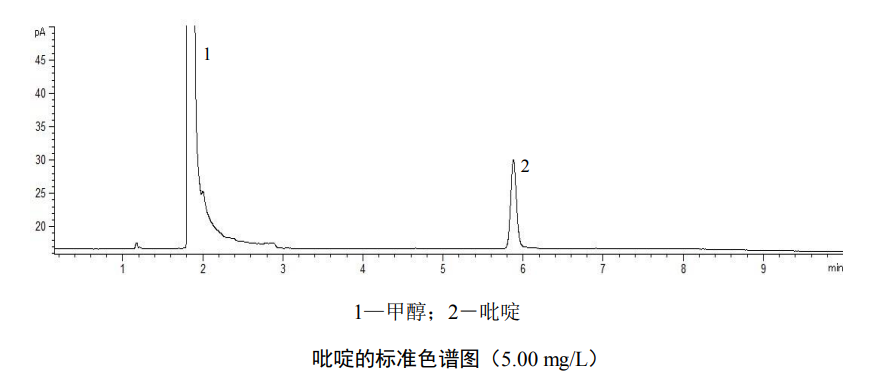
Determination of sample
The measurement of the sample was carried out under the same conditions as the calibration curve. If the pyridine concentration in the sample exceeds the standard curve range, it needs to be diluted and re-measured.
The laboratory blank sample is also measured according to the same steps as the sample determination.
The content of pyridine in the final water sample can be calculated according to P=Pi*D.
In the formula: P—the mass concentration of pyridine in the sample, mg/L;
Pi—The mass concentration of pyridine in the sample obtained from the standard curve, mg/L;
D—the dilution factor of the sample.
The above content comes from 《HJ 1072-2019 Water Quality Determination of Pyridine Headspace/Gas Chromatography》



