In an acidic solution, methanol in water samples is oxidized by potassium permanganate to formaldehyde, which then reacts with chromotropic acid to form a purple compound. The absorbance of this compound is proportional to the content of methanol, the absorbance of the purple compound is measured at a wavelength of 570 nm, and the content of methanol is calculated according to the absorbance.
However, when using the chromotropic acid method to measure methanol, due to the interference of chromaticity, turbidity and other factors, some water samples turn yellow after color development, resulting in high measurement results. Generally, the pretreatment method of distillation can eliminate the interference, but this method should not be used when the collected water samples contain formaldehyde.

Reagents used for testing
1. Sulfuric acid: 1.84g/mL, excellent grade.2. Potassium permanganate solution 1%: Dissolve 1.0 g of potassium permanganate in water, dilute to 100 mL, and store for 15 days.
3. Chromotropic acid solution 0.5%: Weigh 0.5g of chromotropic acid and dissolve it in water, and dilute to 100mL. Put it in a brown bottle to protect from light, refrigerate at 2℃~8℃, it can be stored for 15 days.
4. Sodium sulfite solution 5%: Weigh 5 g of anhydrous sodium sulfite, dissolve in water, make up to 100 mL, and store for 7 days.
5. Methanol: chromatographically pure and above.
6. Methanol standard stock solution: in a 25mL volumetric flask, add 10mL of water first, and accurately weigh the total weight m1 of the container and water. Then add 200ul of methanol with a micro syringe, and use an electronic balance to accurately weigh the total weight m2 of the container and the methanol aqueous solution. The difference between the two weighings (m2-m1) is the mass of methanol, and the solution is adjusted to the mark, and the number of micrograms of methanol in 1.00 mL of the solution is calculated. Commercially available standard solutions can also be used.
7. Methanol standard solution: Dilute the methanol standard stock solution with water to a standard solution of 5ug/mL, which can be stored for 7 days.
Instruments for water testing
1. Spectrophotometer: wavelength range (340nm-1000nm), 20mm glass cuvette.2. Electronic balance: 0.1mg of sense.
3. Electric furnace or constant temperature water bath for experiment.
4. Colorimetric tube with stopper: 50mL.
5. Cooling device: ice water bath.
6. Other glassware commonly used in laboratories.
Water sampling method
Generally, a 500 mL brown narrow-mouth glass bottle is used to collect water samples. The sampling bottle should not be washed with water samples. When sampling, the water samples must be filled with water samples, and there is no gap in the upper part. The water samples were refrigerated at 2°C to 8°C, and the determination was completed within 48 hours.Check the specific steps
Preparation of calibration curve
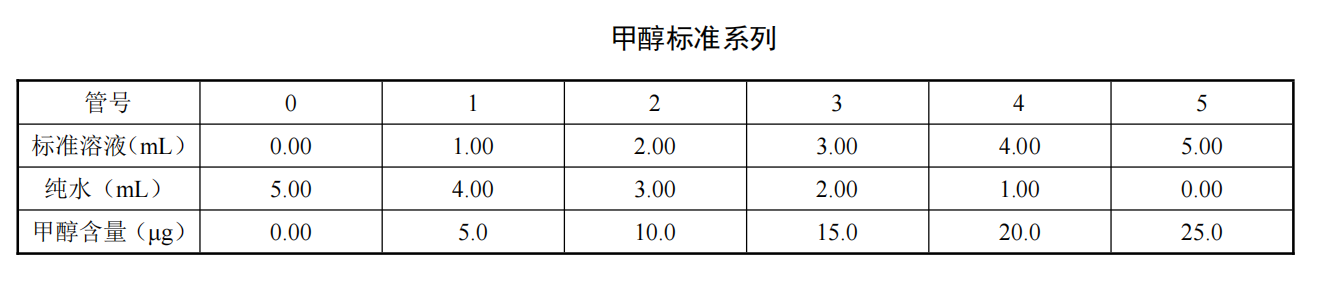
Take six 50mL colorimetric tubes and add 0.00mL, 1.00mL, 2.00mL, 3.00mL, 4.00mL, and 5.00mL methanol standard solutions respectively. Add 5.00mL, 4.00mL, 3.00mL, 2.00mL, 1.00mL, 0.00mL of pure water to each, and prepare according to the methanol standard series chart.Add 0.25mL sulfuric acid and 0.5mL 1% potassium permanganate solution, cover in time and shake well. After standing for 5 minutes, add 5% sodium sulfite solution dropwise until the purple color just fades, then add another drop. Add 0.5 mL of 0.5% color-changing acid solution and shake well. Slowly add 6 mL of sulfuric acid along the wall of the tube, cover it, let it stand for about 30 s, and then shake it up. The solution was heated in a boiling water bath for 15 min, taken out and cooled to room temperature in the dark. Use a 20mm cuvette with water as a reference to measure the absorbance at 570nm. After the standard series of color development, the absorbance remains stable within 24h in a dark place. Taking the methanol content (ug) as the abscissa and the corresponding absorbance as the ordinate, draw a calibration curve, and calculate the slope, intercept and correlation coefficient of the calibration curve.
water sample testing
Take a 5mL water sample (when the volume of the water sample taken is less than 5mL, dilute to 5mL with pure water), and analyze it according to the same steps as the calibration curve drawing. The methanol concentration in the final water sample was calculated according to the corresponding formula.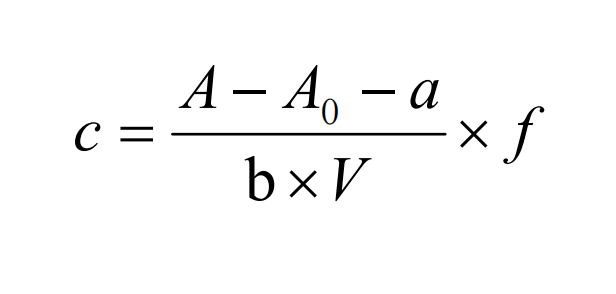
The above methods are from 《DB14/T 2013-2020 Determination of water quality methanol-chromotropic acid spectrophotometry》



