Phytoplankton in surface water usually refers to phytoplankton, which are widely distributed and have many types, such as common cyanobacteria and green algae. Phytoplankton plays a great role in the fish in the water. First, it can be used as a food resource for the fish in the water. Secondly, some phytoplankton can increase the dissolved oxygen content in the water and reduce some salts. However, if there are too many phytoplankton or a large number of reproduction in a short period of time, it will cause damage to the water quality, thus affecting the survival of aquatic organisms. Therefore, through the detection of some phytoplankton, it is possible to judge the current water quality changes.
Phytoplankton in water can usually be detected by filter-microscope counting method. By applying a filter membrane with a certain pore size to the collected water sample, colonies, filaments and single-cell phytoplankton are trapped on the filter membrane. After the transparent treatment, the number and type of phytoplankton in the tested water sample are obtained by microscopic examination under the microscope, so as to know the water quality of the water sample.

Detection reagents and materials
1. Iodine (I2).2. Potassium iodide (KI).
3. Formaldehyde solution: 37%-40%.
4. Lugol's iodine solution:
Weigh 60g of potassium iodide and dissolve it in 100ml of water, then add 40g of iodine, stir well to dissolve it completely, add water to make up to 1000ml, transfer to a brown ground-mouth glass bottle, and store at room temperature away from light.
5. Microscope immersion oil: 1.515 or 1.518, with a transparent glass drop stick.
6. Filter membrane: mixed cellulose ester membrane, diameter 25mm, pore size 0.45um-3um.
Instruments required for testing
1. Plankton net No. 25: the diameter of the mesh is 0.064mm, the net is conical, the net mouth is set on the copper ring, and the bottom end of the net has a water outlet switch piston.2. Qualitative sampling bottle: 30ml-100ml wide mouth polyethylene bottle.
3. Water collector: stainless steel or plexiglass material, cylindrical. Capacity and depth specifications to meet sampling requirements.
4. Quantitative sampling bottle: 1L ~ 2L wide mouth polyethylene bottle.
5. Refrigerators.
6. Vacuum filtration device: including funnel (diameter 25mm, volume 200ml), porous polyester filter membrane support plate, collection bottle, vacuum pump (relative vacuum degree ≤-17kPa, adjustable).
7. Glass slide: 25mm×76mm.
8. Cover glass: 25mm×25mm.
9. Teethless tissue forceps.
10. Slide drying plate: suitable for slides of the same specification.
11. Oven: 70℃±2℃, the temperature is adjustable.
12. Upright biological microscope or inverted biological microscope: objective lens 4×, 10×, 20×, 40×; eyepiece 10× or 15×.
13. Stage micrometer: also known as stage micrometer, 1mm/100DIV, the division value is 0.01mm.
14. Whipplegrid: A large square grid with a side length of 7mm-10mm is divided into 100 identical square middle grids, and the 1 middle grid in the center is further divided into 25 identical small square grids .
15. Counter.
16. Common laboratory equipment.
Water sample collection and preservation
Qualitative water sample collection
Qualitative samples were collected using plankton net 25. Turn off the water outlet piston switch at the bottom of the plankton net, reciprocate in a "∞" shape at a speed of 20cm/s-30cm/s from the surface of the water to a depth of 0.5m, and slowly drag it for about 1min-3min, until there are phytoplankton in the net. , lift the plankton net from the water surface, and the water in the net is naturally filtered out through the mesh holes. When there is a little water sample (5ml-10ml) left at the bottom, move the bottom outlet into the qualitative sampling bottle, and open the bottom piston switch to collect qualitative samples. When collecting stratified samples, use No. 25 plankton net to filter specific water layer samples, and other steps are the same as collecting surface samples. Clean the plankton net in time after the qualitative sample collection is completed. After the samples were collected, they were placed in a refrigerated box for transport away from light.If there is a technical specification for qualitative sample collection, follow the relevant requirements of the technical specification.
After the qualitative sample is collected, Lugol's iodine solution should be added immediately for fixation, and the dosage is 1.0%-1.5% of the volume of the water sample. Microscopic biopsy samples were not fixed with Lugol's iodine solution. Qualitative samples can be stored for 3 weeks at room temperature and protected from light; 12 months at 1°C-5°C in the dark. In vivo samples can be stored for 36h under refrigerated storage at 4℃-10℃ and protected from light.
Quantitative water sample collection
Use a water collector to collect 1 L-2 L samples into quantitative sampling bottles. If the transparency of the water body is high and the number of phytoplankton is small, the sampling volume should be increased as appropriate. After the quantitative sample collection is completed, the quantitative sampling bottle should not be full so that it can be shaken well.Immediately after the quantitative water samples were collected, Lugol's iodine solution was added for fixation, and the dosage was 1.0%-1.5% of the volume of the water samples. Lugol's iodine solution can also be added to the quantitative sampling bottle in advance and brought to the field for use. Quantitative water samples can be stored for 3 weeks at room temperature in the dark; 12 months in the refrigerator at 1 ℃-5 ℃ in the dark.
During the preservation process of water samples, the oxidation degree of Lugol's iodine solution should be checked every week. If the color of the water sample becomes lighter, an appropriate amount of Lugol's iodine solution should be added to the water sample until the color of the water sample returns to yellow. brown.
Detection steps
First of all, it is required to complete the pre-inspection and loading, and judge the density of phytoplankton in the water sample, which is convenient for the subsequent analysis of the water sample. Secondly, before each sampling, fully mix the water sample by inverting it upside down at least 30 times, and the mixing action should be light.Estimate the density of phytoplankton according to the pre-check, adjust the filtration volume of the water sample, and finally make about 40-105 phytoplankton cells on the filter membrane. Refer to the relevant charts for the recommended filtration volumes for water samples at different pre-check estimated density levels.

water sample filtration
Use a graduated cylinder or a pipette to quickly measure an appropriate volume of water sample, add the water sample to the funnel of the vacuum filtration device equipped with a filter membrane, let it stand for 2min-3min, vacuum filtration, and close when there is a 0.5cm liquid layer in the funnel Vacuum pump, make the remaining liquid pass through the funnel completely, do not dry the filter membrane.Mounting preparation
After the sample is filtered, remove the filter membrane with toothless tissue tweezers, keep the phytoplankton-retaining side facing up, and place it on a glass slide with 2 drops of microscope immersion oil, and then use a transparent glass drop stick to drop 2 drops on the filter membrane. Immerse the microscope in oil, put the slides into the slide plate, put them in the oven, and heat them at 70℃±2℃ for 2h. After 2 hours, take out the slide plate and observe whether the filter membrane is transparent. If it is transparent, add 2 drops of microscope immersion oil on the filter membrane, cover with a cover glass, and complete the preparation. If the filter membrane is not transparent after heating for 2h, prolong the heating time, not more than 24h. Do not disturb the filter while covering with a coverslip.
Qualitative water sample testing
Qualitative samples were observed under a microscope to identify the species of phytoplankton. The dominant species should be identified as species, other species should at least be identified as genus.Quantitative water sample testing
Microscope calibrationCalibrate the microscope before counting to determine the area of the counting field (Whipple field or eyepiece field). Microscope calibration tools include a stage micrometer and a Whipple eyepiece reticle.
Microscope count
Refer to the relevant charts for the recommended visual field categories and the number of counted visual fields for samples with different density levels. Place the mount on the microscope stage and perform microscopic counts using the Whipple field of view or the eyepiece field of view. For sample counting at lower density levels, it is recommended to select the eyepiece field of view field of view for counting. According to the size of phytoplankton cells, choose eyepiece 10×, objective 20× or eyepiece 10×, objective 40× magnification for microscopy, and record the species and quantity of phytoplankton in each field of view.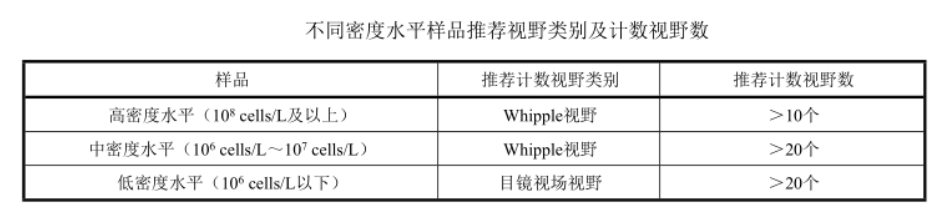
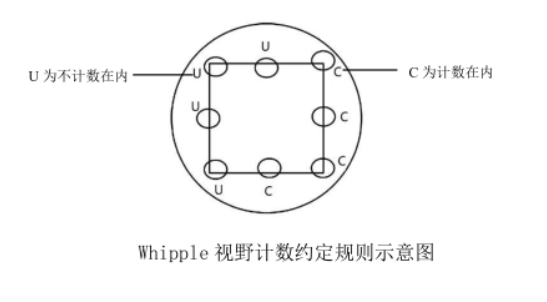
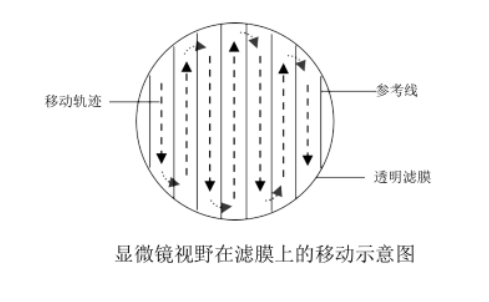
Whipple visual field counting rules: Algae on the lower and right boundary lines in the visual field are counted into the total number, and algae on the upper and left boundary lines are not counted in the total number, as shown in the schematic diagram of the Whipple visual field counting convention rules. If larger individuals such as filaments significantly cross the boundaries of two or more grids, they should be counted individually under low magnification, and then added to the total. Damaged cells were not counted. When counting, move the microscope stage slowly, avoid repeated sampling in one area, and ensure that the field of view of the upper, lower, left, right and middle areas of the filter is sampled. The moving direction of the microscope field of view on the filter membrane is shown in the schematic diagram of the movement of the microscope field of view on the filter film.
The cell density of phytoplankton in the final water sample can be calculated according to the relevant formula.
The above content comes from 《HJ 1215-2021 Water Quality Determination of Phytoplankton Filter Membrane-Microscopic Counting Method》



