The azide in the water sample is heated and converted into azide acid in an acidic medium, which is distilled out with water vapor and absorbed by sodium hydroxide solution, and the azide ion reacts with ferric ion to form a brown-red complex. Its absorbance was measured at 454 nm. In a certain concentration range, its absorbance is proportional to the azide ion content.

Reagents used for testing
1. Sodium hydroxide.2. Hydrochloric acid: 1.19g/ml.
3. Ferric perchlorate: chemically pure.
4. Sodium azide: 99.5%.
5. Ammonium sulfamate.
6. Sodium hydroxide solution: 400mg/L.
Weigh 0.40g of sodium hydroxide, dissolve it with a small amount of water, transfer it to a 1000ml volumetric flask, dilute it to the mark, shake well, and store in a polyethylene bottle.
7. Sodium hydroxide solution: 40mg/L.
Pipette 100ml of sodium hydroxide solution into a 1000ml volumetric flask, dilute with water to the mark, shake well, and store in a polyethylene bottle.
8. Hydrochloric acid solution: 1.0mol/L.
Pipette 8.3ml of hydrochloric acid into a 100ml volumetric flask, dilute with water to the mark, shake well, and store in a ground glass bottle.
9. Hydrochloric acid solution: 0.1mol/L.
Pipette 10.0ml of hydrochloric acid solution into a 100ml volumetric flask, dilute with water to the marked line, shake well, and store in a ground glass bottle.
10. Ferric perchlorate solution: 50g/L.
Weigh 5.00g of ferric perchlorate, dissolve it with 20ml of hydrochloric acid solution, transfer it to a 100ml volumetric flask, dilute with water to the mark, shake well, and store in a brown ground-mouth glass bottle. Refrigerated below 4°C and protected from light, it can be stored for three months.
11. Ammonium sulfamate solution: 150g/L.
Weigh 15.00g of ammonium sulfamate, dissolve with a small amount of water, transfer to a 100ml volumetric flask and dilute to the mark, shake well. Ready to use.
12. Standard stock solution of sodium azide: 1.00g/L.
Accurately weigh 0.1548g of sodium azide, dissolve it with a small amount of sodium hydroxide solution, transfer it to a 100ml volumetric flask, dilute it with sodium hydroxide solution to the mark, shake well, and store in a polyethylene plastic bottle. Refrigerated below 4°C and protected from light, it can be stored for three months.
13. Standard solution of sodium azide: 100mg/L.
Pipette 10.0ml of sodium azide standard stock solution into a 100ml volumetric flask, dilute with sodium hydroxide solution to the mark, and shake well. Ready to use.
14. Glass beads: 4mm~6mm.
Instruments used for testing
1. Visible spectrophotometer: with 10mm cuvette.2. Power adjustable electric furnace.
3. Distillation device: all-glass still (with 500ml distillation flask, condenser tube), receiving bottle, distillate conduit.
4. Colorimetric tube with stopper: 10ml.
5. Sampling bottle: brown glass bottle.
6. General laboratory instruments and equipment.
water sample preparation
Water samples should be collected in accordance with the relevant collection standards, and the collection volume should not be less than 500mL. After collection, an appropriate amount of sodium hydroxide can be added to adjust the pH of the water sample to 9-11, and the water samples should be stored at room temperature in the dark, and the analysis will be completed within 7 days.Measure 150ml of water sample, transfer it into a distillation flask, add 5.0ml of ammonium sulfamate solution, put in several glass beads, insert the distillate catheter into the receiving bottle containing 10.0ml of sodium hydroxide solution, open the condensed water, Add 2.0 ml of hydrochloric acid solution to the distillation flask, and quickly close the stopper of the distillation flask. Turn on the electric furnace with adjustable power, slowly raise the temperature, control the distillate to distill out at a rate of 2ml/min to 3ml/min, and stop heating when the distillate in the receiving bottle is close to 100ml. Rinse the inner wall of the condensing tube and the distillate conduit with a small amount of water, and pour it into the receiving bottle, and set the volume to 100ml, to be tested. The distillation process should be controlled within 30min-45min.
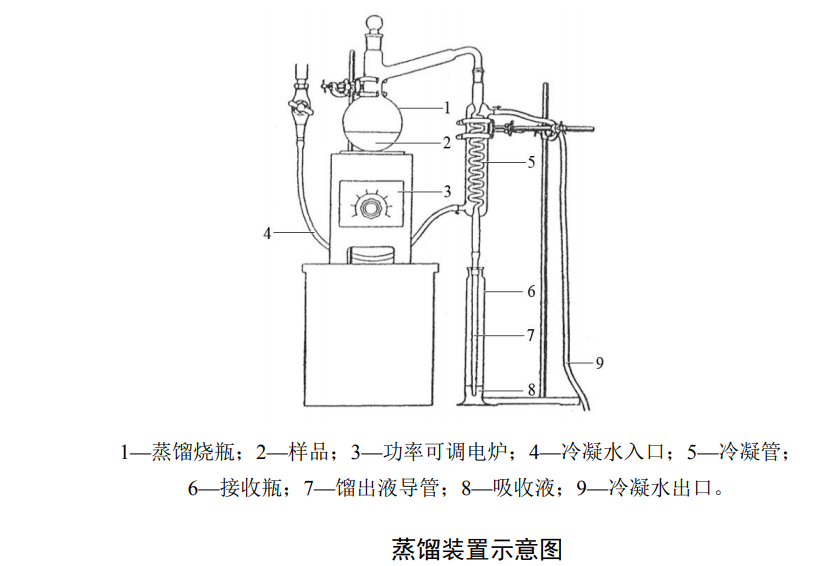
Water sample testing process
Create a calibration curveTake 6 colorimetric tubes with stopper, add 0ml, 0.05ml, 0.20ml, 0.50ml, 1.00ml, 1.50ml of sodium azide standard solution, and immediately add sodium hydroxide solution to 10.0ml to prepare azide root Standard series solutions with mass concentrations of 0mg/L, 0.50mg/L, 2.00mg/L, 5.00mg/L, 10.0mg/L and 15.0mg/L respectively. Add 0.5ml of ferric perchlorate solution to each colorimetric tube, close the stopper tightly, and shake well. At the wavelength of 454nm, use a 10mm cuvette with water as a reference to measure the absorbance of each standard series solution, and the measurement is completed within 20min.
Take the mass concentration of azide (mg/L) in each standard series solution as the abscissa, and its corresponding absorbance after subtracting the reagent blank (zero concentration) as the ordinate to establish a calibration curve.
Sample determination
Pipette 10.0ml of the sample into a colorimetric tube with a stopper, and perform the measurement of the sample according to the same steps as establishing the calibration curve. Then follow the same procedure for blank test. The final azide content can be calculated using the relevant formula.



