The trihalomethanes generation potential refers to the maximum amount of trihalomethanes that can be produced in water samples after reacting with chlorine for a long enough time under the condition that sufficient chlorine is added. The detection principle is to add enough chlorine into the water sample, allow the precursor of trihalomethanes to react to produce trihalomethanes in a sufficiently long time, and detect the amount of trihalomethanes generated by headspace gas chromatography to characterize the trihalomethanes generation potential.

Reagents required for testing
1. Nitrogen: purity ≥99.999%.2. High purity water: meet the requirements of GB/T33087.
3. Sodium hydroxide: analytically pure.
4. Anhydrous potassium dihydrogen phosphate: analytically pure.
5. Methanol: chromatographically pure.
6. Ascorbic acid: analytically pure.
7. Hydrochloric acid: 1.19 g/mL, analytically pure.
8. Sodium hypochlorite: available chlorine ≥ 10%.
9. Trichloromethane standard solution: 1000mg/L, solvent is methanol, certified reference material.
10. Dichloromonobromomethane standard solution: 2000mg/L, solvent is methanol, certified reference material.
11. Monochlorodibromomethane standard solution: 2000mg/L, solvent is methanol, certified reference material.
12. Tribromomethane standard solution: 2000mg/L, solvent is methanol, certified reference material.
13. Trihalomethane standard stock solution: ρ (trichloromethane)=4.0mg/L, ρ (dichloro-bromomethane)=2.0mg/L, ρ (monochlorodibromomethane)=2.0mg/L, ρ (trichlorodibromomethane)=2.0mg/L methyl bromide) = 10 mg/L. Accurately pipette 40 μL of chloroform standard solution (4.9), 10 μL of dichloro-bromomethane standard solution (4.10), 10 μL of chlorodibromomethane standard solution (4.11), and 50 μL of chloroform standard solution (4.12) into a 10-mL volumetric flask, Make up to volume with methanol and mix.
14. Trihalomethane standard solution: ρ(trichloromethane)=0.4mg/L, ρ(dichloro-bromomethane)=0.2mg/L, ρ(monochlorodibromomethane)=0.2mg/L, ρ(trichlorodibromomethane)=0.2mg/L methyl bromide) = 1.0 mg/L. Accurately pipette 1mL of the standard stock solution of trihalomethane into a 10mL volumetric flask, dilute to the mark with methanol, and mix well.
15. Sodium hydroxide solution (40g/L): Weigh 20g of sodium hydroxide, dissolve in high-purity water, and dilute to 500mL.
16. Hydrochloric acid solution (1+9): Slowly add hydrochloric acid to high-purity water to prepare a hydrochloric acid solution with a volume ratio of 1:9.
17. Phosphate buffer: dissolve 68.1 g of anhydrous potassium dihydrogen phosphate and 11.7 g of sodium hydroxide in high-purity water, and make up to 1 L. Store refrigerated below 4°C.
18. Sodium hypochlorite solution: containing 5g/L of available chlorine, diluted with sodium hypochlorite and high-purity water, ready for use.
Instruments required for testing
1. Gas chromatograph: equipped with electron capture detector.2. Chromatographic column: HP-5 (30m×320um×0.25um) or other equivalent chromatographic column.
3. Headspace sampler.
4. Headspace sampling bottle: 60 mL (fitted with Teflon silicone gasket and plastic screw cap seal), bake at 120 °C for 2 h before use.
5. Headspace vial: 20 mL, bake at 120 °C for 2 h before use.
6. Headspace bottle sealing cap: equipped with a 20 mL headspace bottle for single use.
7. Volumetric flasks: 10 mL and 100 mL.
8. Micro syringes: 100ul, 500ul and 1 000ul.
9. Pipette: 10 mL.
10. Chlorine colorimeter.
11. Constant temperature incubator.
water sample preservation
After collection, the water samples should be sent back to the laboratory for analysis as soon as possible. If they cannot be analyzed in time, they can be refrigerated and stored below 4°C. The background samples of trihalomethanes should be measured within 24 hours, and the generation potential of trihalomethanes should be measured within 7 days. The sample storage area is free of organic interference.water sample preparation
60mL water samples were taken into two headspace sampling bottles, namely bottle I and bottle II.
Bottle I is the bottom water sample, first add 0.3g-0.5g ascorbic acid to the headspace bottle during sampling. Pipette 10mL of water sample from bottle 1 into a 20mL headspace bottle for testing.
Add 60uL of phosphate buffer to bottle II, adjust pH=8.0±0.2 with sodium hydroxide solution or hydrochloric acid solution, add 80uL of sodium hypochlorite solution, put it in a constant temperature incubator at 20°C for 24h reaction in the dark, use a residual chlorine colorimeter The residual chlorine concentration should be determined to be (1.0 ± 0.4) mg/L, and ascorbic acid was added to terminate the reaction. If the residual chlorine is not within this range, repeat the experiment. Use a pipette to transfer 10 mL of the water sample in bottle II into a 20 mL headspace bottle for testing.
Detection steps
Headspace Sampler ConditionsHeater temperature: 60°C.
Loop temperature: 70°C.
Transfer line temperature: 80°C.
Heating time: 20min.
Chromatographic analysis conditions
Injection volume: 1.0mL.
Injection method: split, split ratio of 20:1.
Injection port temperature: 200°C.
Detector temperature: 300°C.
Column temperature program: 35°C for 5 min, then ramp to 50°C at 1°C/min.
Column flow: 1.0 mL/min.
Makeup flow: 20mL/min.
Septum purge flow: 3.0 mL/min.
Plotting the calibration curve
Take six 100mL volumetric flasks, add an appropriate amount of high-purity water, and then add 100uL, 200uL, 400μL, 600uL, 800uL, and 1000uL of standard trihalomethanes to 6 volumetric flasks, and make up to the mark with high-purity water to prepare trihalomethanes The mixed standard series of chloroform, the concentration of chloroform is 0.0004mg/L, 0.0008mg/L, 0.0016mg/L, 0.0024mg/L, 0.0032mg/L and 0.0040mg/L; the concentration of dichloromonobromomethane is 0.0002mg/L , 0.0004mg/L, 0.0008mg/L, 0.0012mg/L, 0.0016mg/L and 0.0020mg/L; chlorodibromomethane concentrations were 0.0002mg/L, 0.0004mg/L, 0.0008mg/L, 0.0012mg/L L, 0.0016mg/L and 0.0020mg/L; bromomethane concentrations were 0.001mg/L, 0.002mg/L, 0.004mg/L, 0.006mg/L, 0.008mg/L and 0.010mg/L. Pipette 10 mL of mixed standard series solutions into headspace vials, and measure them in order of concentration from small to large. Draw the calibration curve with the mass concentration of trihalomethane as the abscissa and the peak area as the ordinate.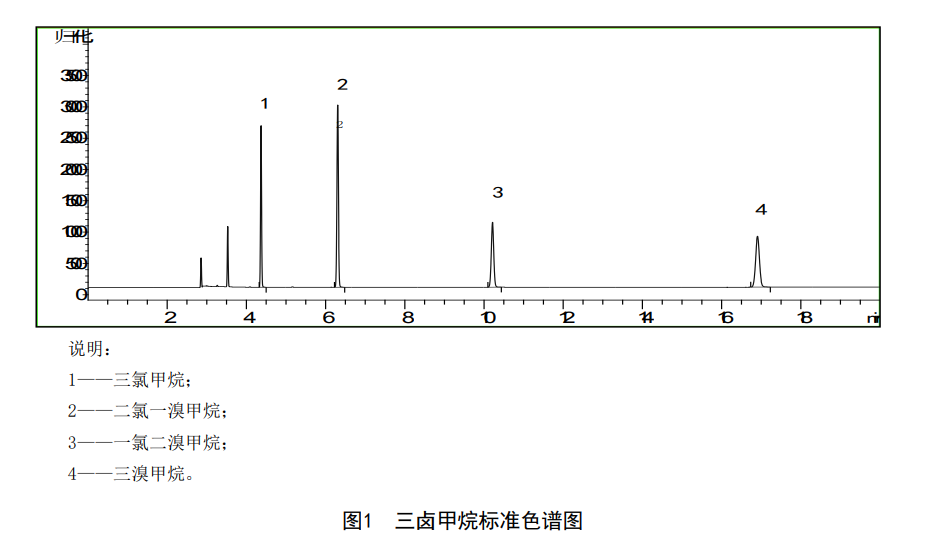
water sample testing
The prepared water samples were measured according to the drawing procedure with the calibration curve. Qualitative analysis was performed based on the retention time of the sample, and the relative deviation of the retention time was within ±3%. Quantitative analysis can determine the content of each component in the water sample through the calibration curve based on the peak area of each component. The generation potential of trihalomethanes is the difference between the concentration after chlorination reaction for 24 h and the background concentration of the sample, which can be calculated according to the relevant formula.The above content comes from 《DB37/T 4147—2020 Determination of Trihalomethane Generation Potential in Water Quality-Headspace-Gas Chromatography》



